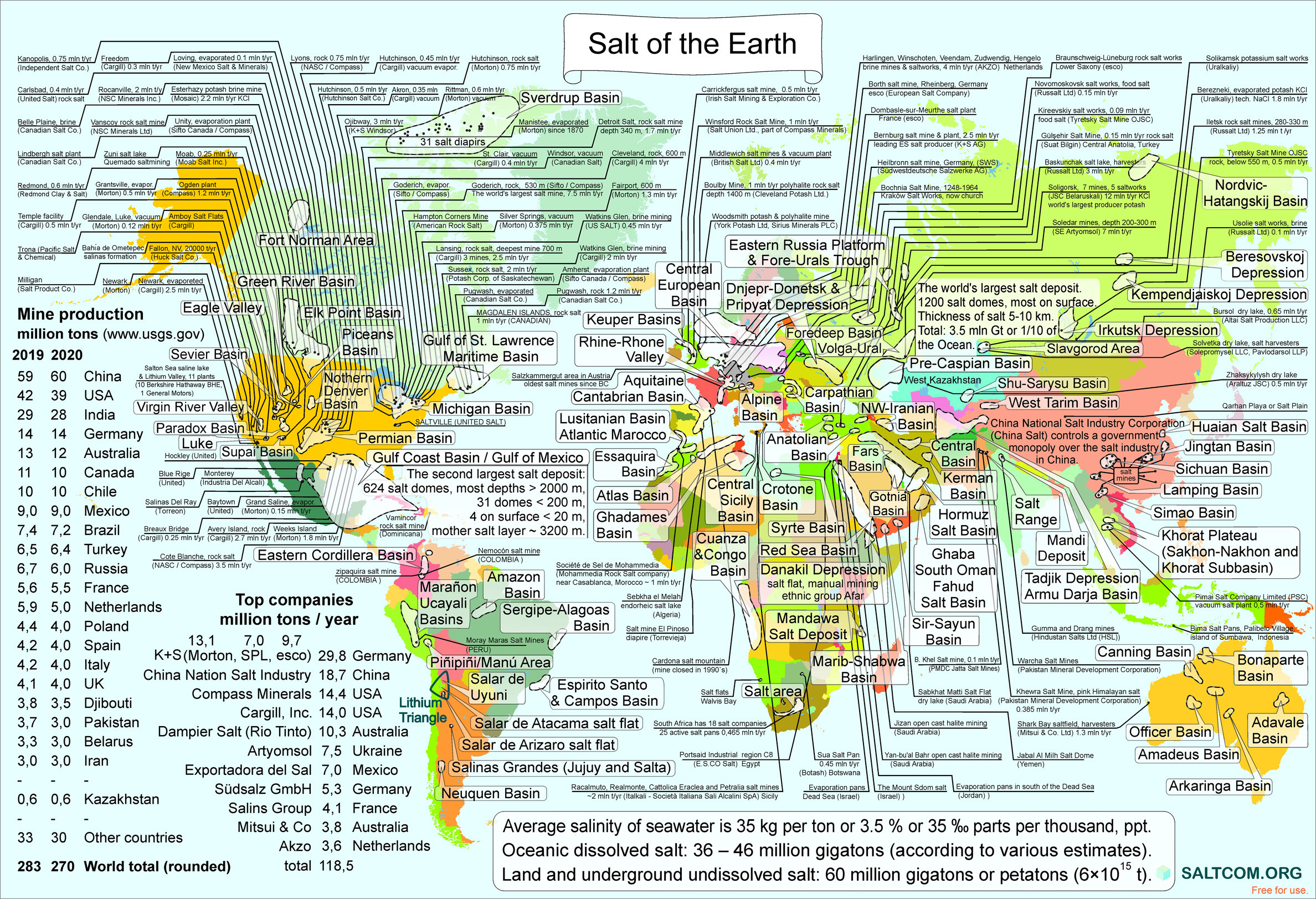
Kazakhstan`s scientists
proposed to salt the Ocean
Introduction Carbonate-Borate System MATHEMATICAL CHEMISTRY Welcome to Kazakhstan Global life hacks Salt of the Earth Discussions Vision

Introduction Carbonate-Borate System MATHEMATICAL CHEMISTRY Welcome to Kazakhstan Global life hacks Salt of the Earth Discussions Vision
Size of carbon reservoirs on the Earth
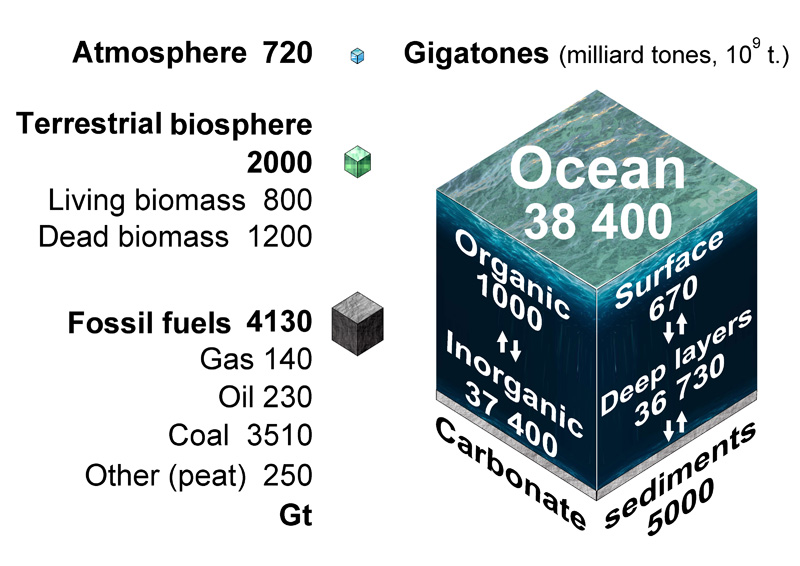
As you can see in this diagram, most carbon stocks are concentrated in the deep ocean and they are inorganic. The dissolved inorganic carbon (DIC) in the surface layer is exchanged rapidly with the atmosphere CO2, maintaining equilibrium Henry's law.
The deep ocean contains far more carbon as ions, it is the largest pool of actively cycled carbon in the world, containing 50 times more than the atmosphere. The ocean depth is the core of the carbon cycle system.
More than 99 % of inorganic carbon is in the form of two ions:
carbonate CO32– and bicarbonate HCO3–.
A fourth form is H2CO3 (true carbonic acid), the concentration of which is much lower (~ 0.3 %) than aqueous CO2. Carbonic acid rapidly dissociates into free hydrogen ion and bicarbonate.
CO2 (aq) + H2O ⇔ H2CO3 ⇔ HCO3– + H+ ⇔ CO32– + 2H+
The balance of these carbonate species with hydrogen ions is a nonlinear thermodynamically coupled system and depends on factors such as temperature, pressure and salinity of seawater.
DIC can be converted to particulate inorganic carbon by biological or abiotic precipitation in the carbonate minerals: calcite CaCO3, magnesite MgCO3 etc.
The main producers of carbonate sediments in seawater are unicellular organisms such as foraminifera and coccolithophore.
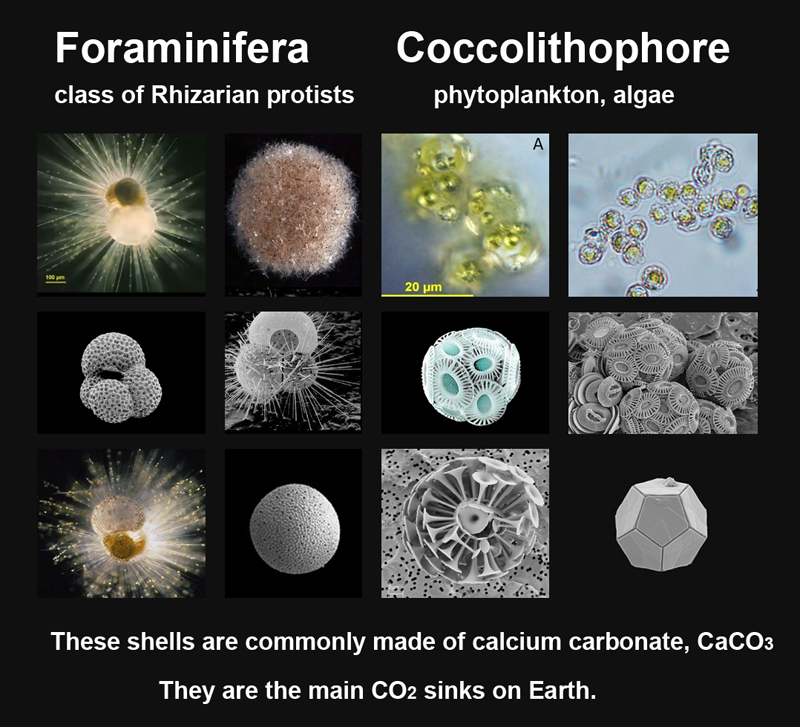
There are so-called "foraminiferal calcite rains" in the ocean, which form calcareous silts, or also called foraminiferal ooze, are carbonate sediments that cover about 50 % of the seabed of the World Ocean.
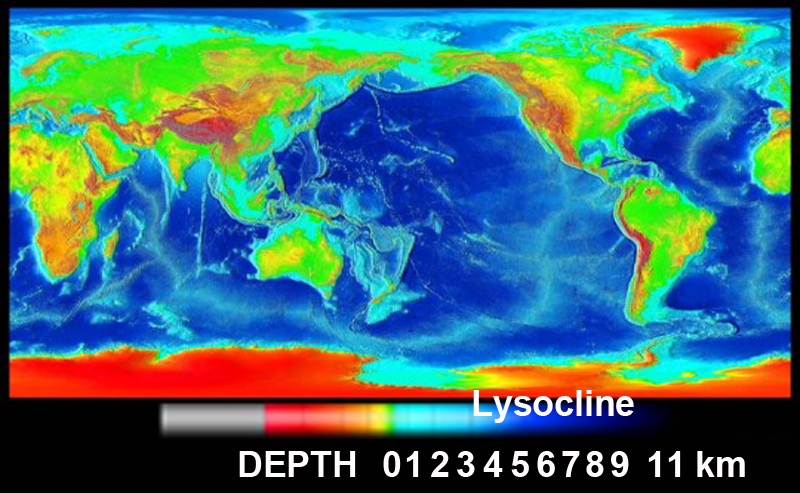
If we compare the ocean depth map,
and the carbonate sediment map
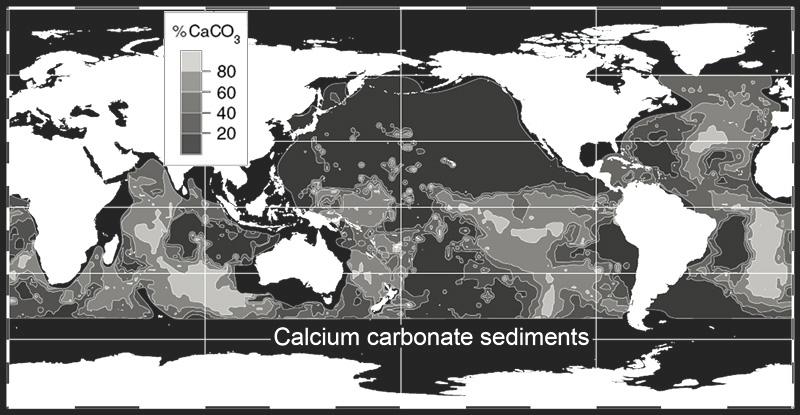
we can see a correlation between surface salinity and bottom sediments. Depths below the lysocline ~3.5 km (dark blue on depth map) preclude carbonate accumulation due to high pressure and low temperature effects on CaCO3 dissolution.
Tops of seamounts are covered with carbonate sediments, like snow-capped peaks on land.
Seawater is weakly alkaline, and the ocean chemistry describes the charge balance of all its elements and total alkalinity, TA, which includes carbonate and borate ions:
TA = [CO3– ] + 2[CO23– ] + [OH– ] + [H+] + [B(OH)4– ]
+ minor components
Due to the fact that boron is present in the marine at lower concentrations, the borate system plays a much smaller role than the carbonate system, but its contribution is not so small as to be neglected.
The influence of the rest of the components ([PO43–−], [HPO42–], [SiO(OH)3–], [HSO4–]) is minimal and is within the statistical limits of the measurement error.
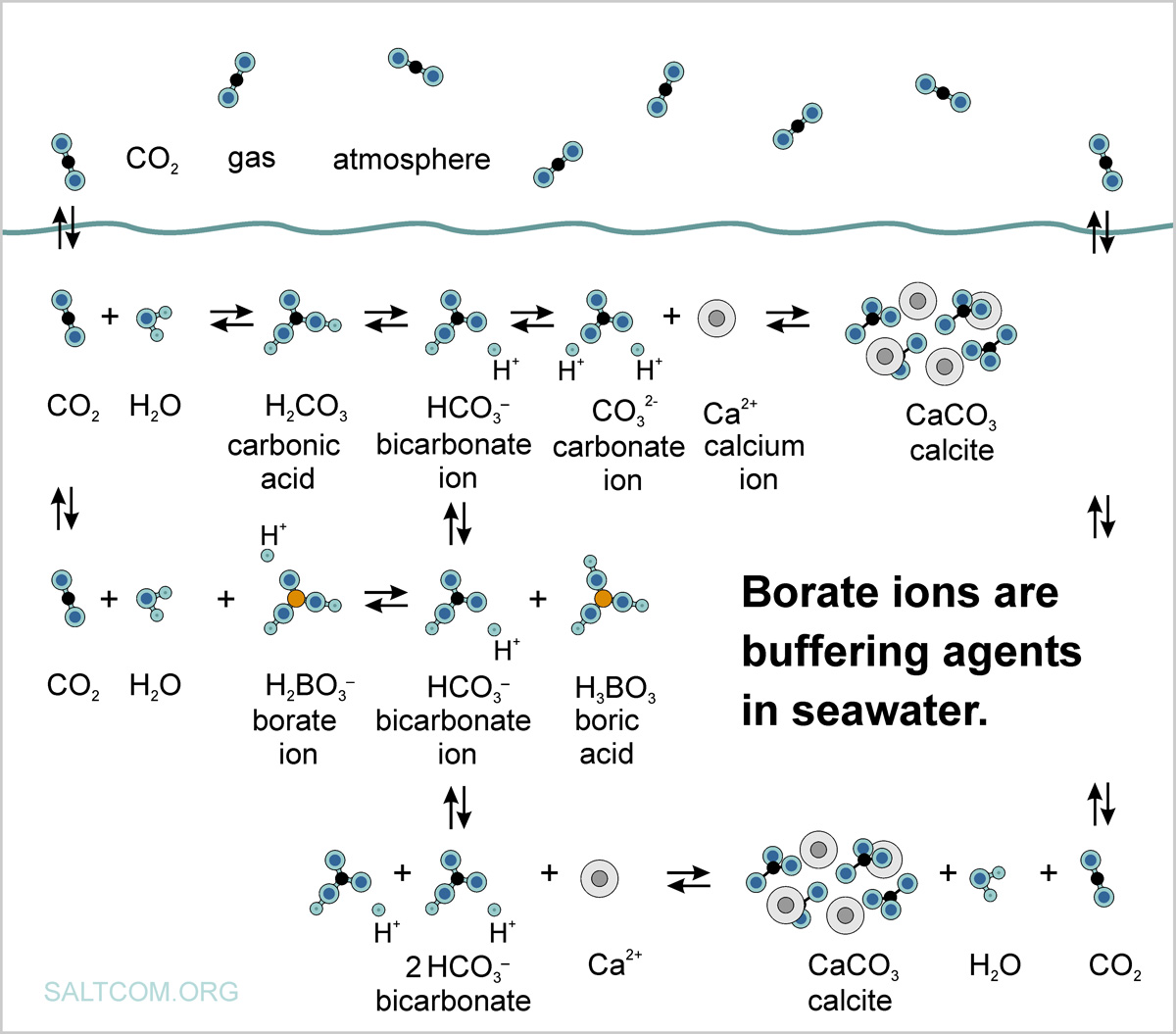
Seawater, as an electrolyte containing several different ions, is a natural buffer solution. The thermodynamic conjugation buffer systems are a mixture of an acid (proton donor) and a base conjugated (proton acceptor), that is, particles differing by proton H+.
When calcite CaCO3 is precipitated from a supersaturated solution, the excess or deficiency of dissolved Ca2+ should be equal to the difference in TA in the initial and final states.
The decrease in carbonate alkalinity due to the release of CaCO3 from the solution is partially compensated by the appearance of HCO3– ions in the solution only due to a decrease in the borate component.
 You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
The concentration of borate ions in seawater depends on pH and increases with its increase, forming a kind of fixing wedge or cline that supports balance, which is associated with the thermodynamic concept of degrees of freedom. We called this the "third leg effect".;
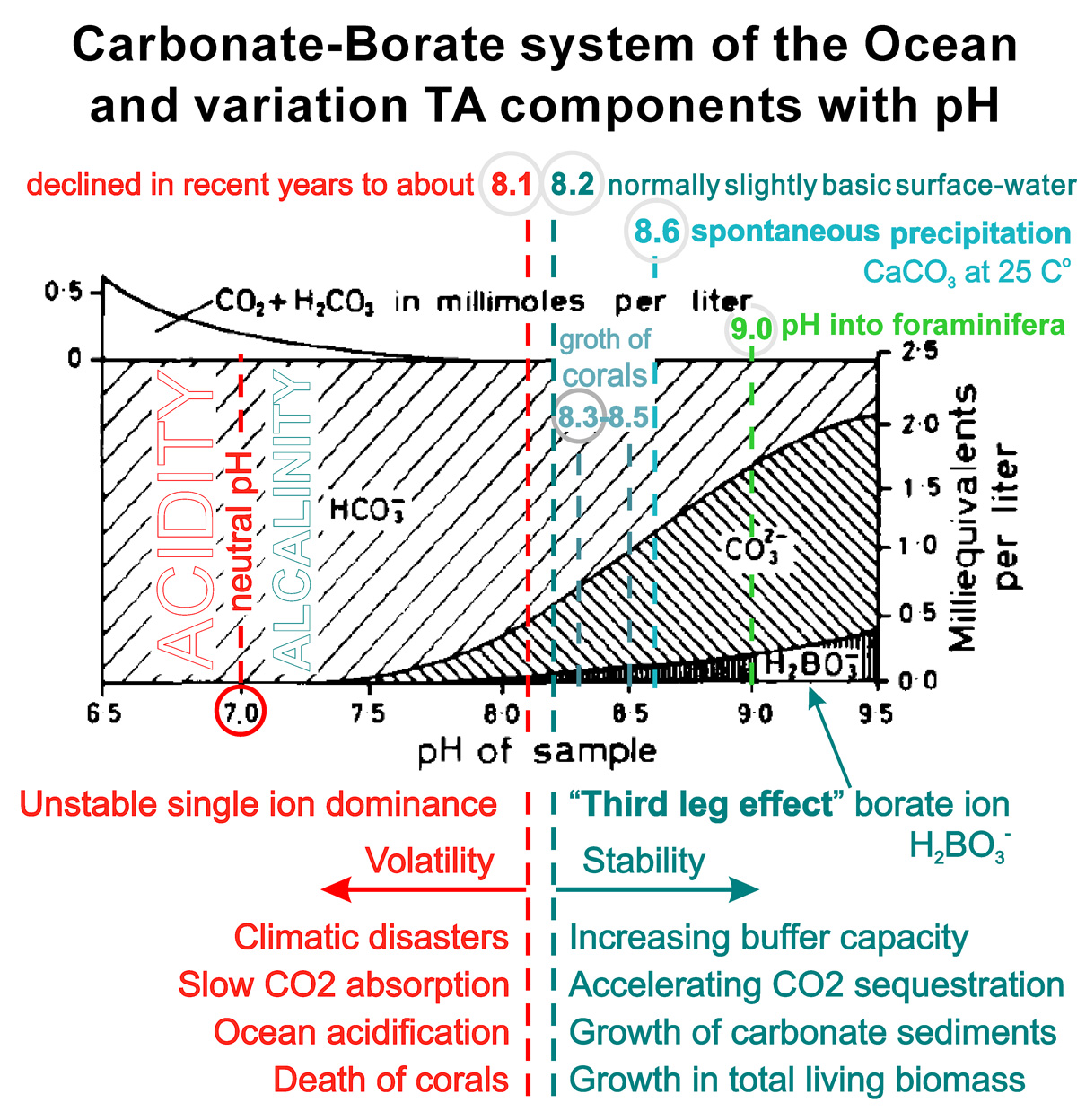
Seawater, being alkaline, contains an excess of base that can be balanced with a weak acid such as H2CO3. Сarbonic acid is said to be weak rather than "dilute" because it only partially dissociates when dissolved in water. This partiality, together with a "borate-cline", constitutes a buffer balance of positive and negative charges in seawater, the sum of which is zero.
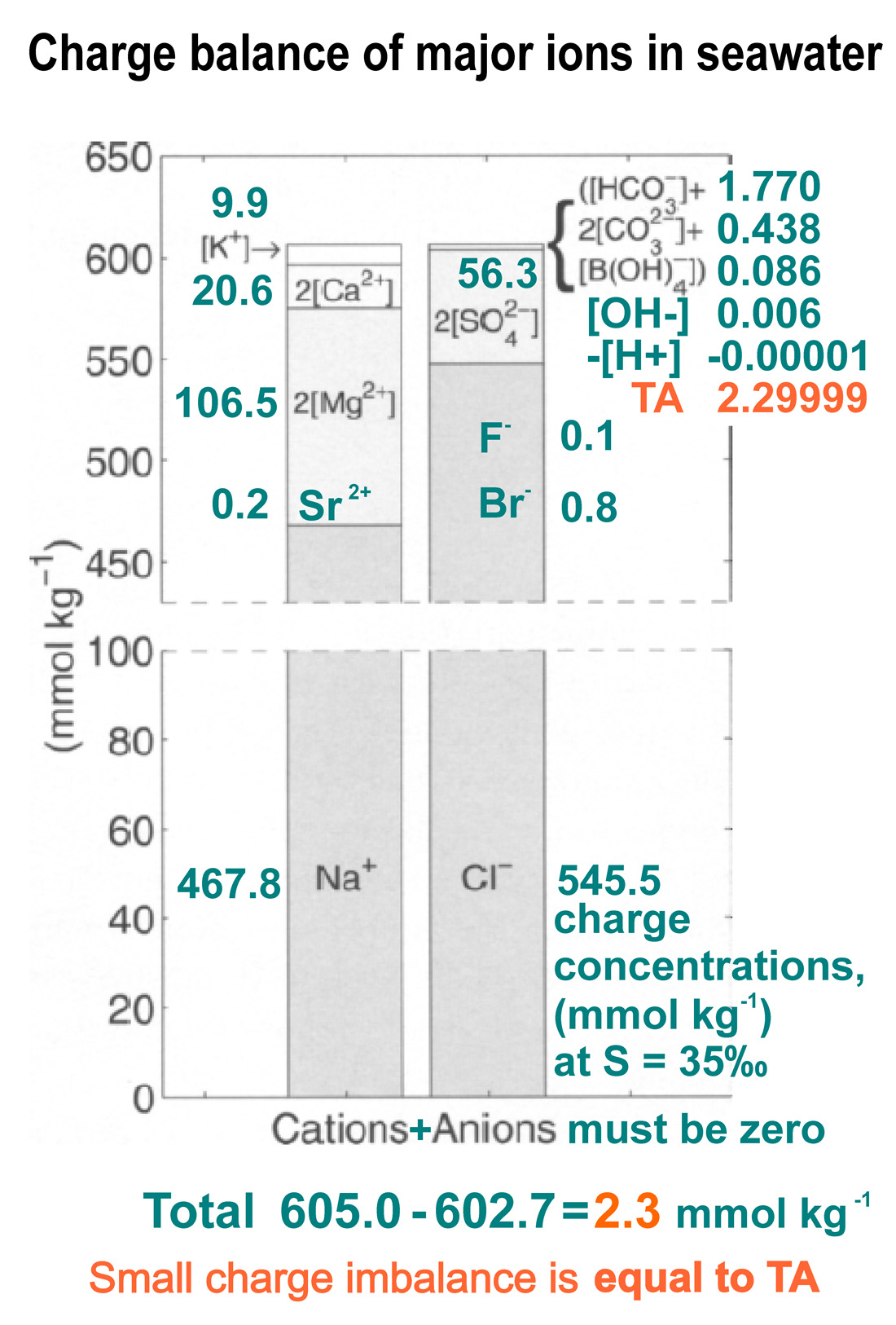
The small excess charge of the conservative cations over anions is mainly balanced by the sum represents the most important contribution to TA – carbonate, bicarbonate, and borate ions.
Total alkalinity remains constant with absorption or desorption of CO2, but change with salinity.
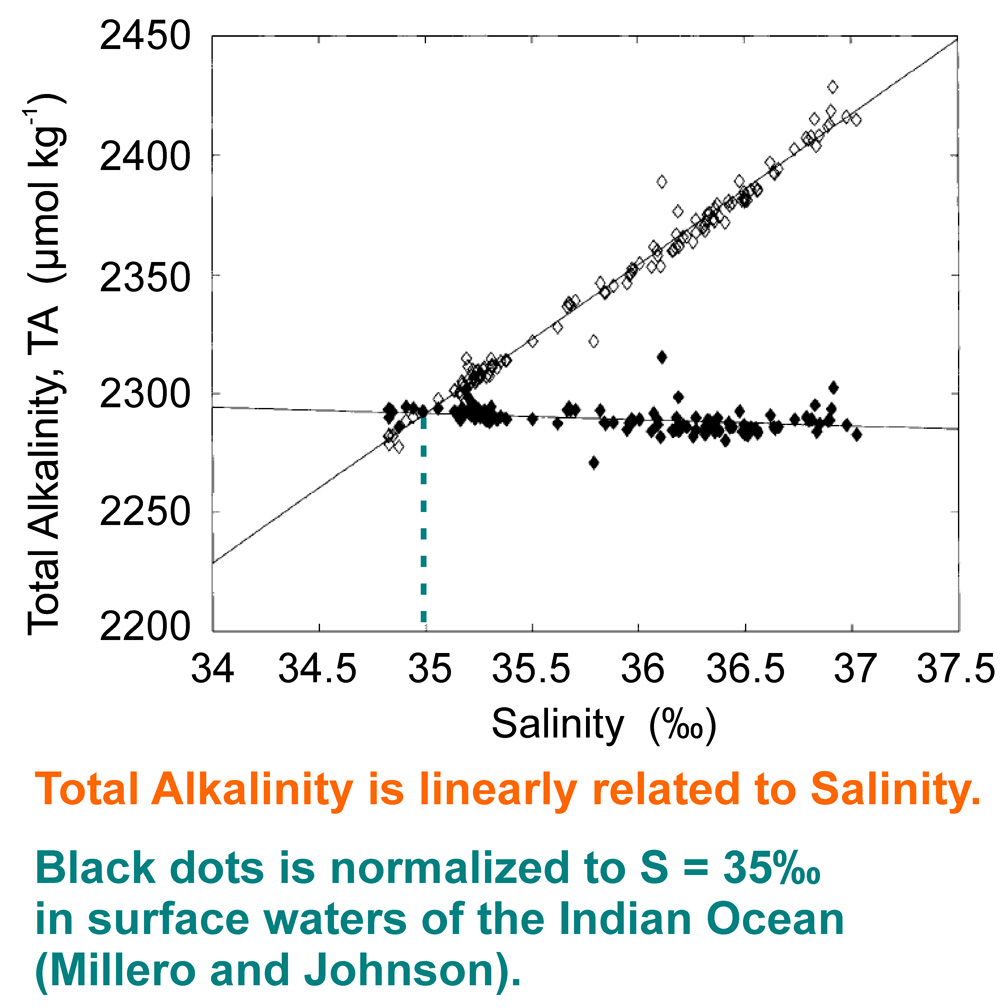
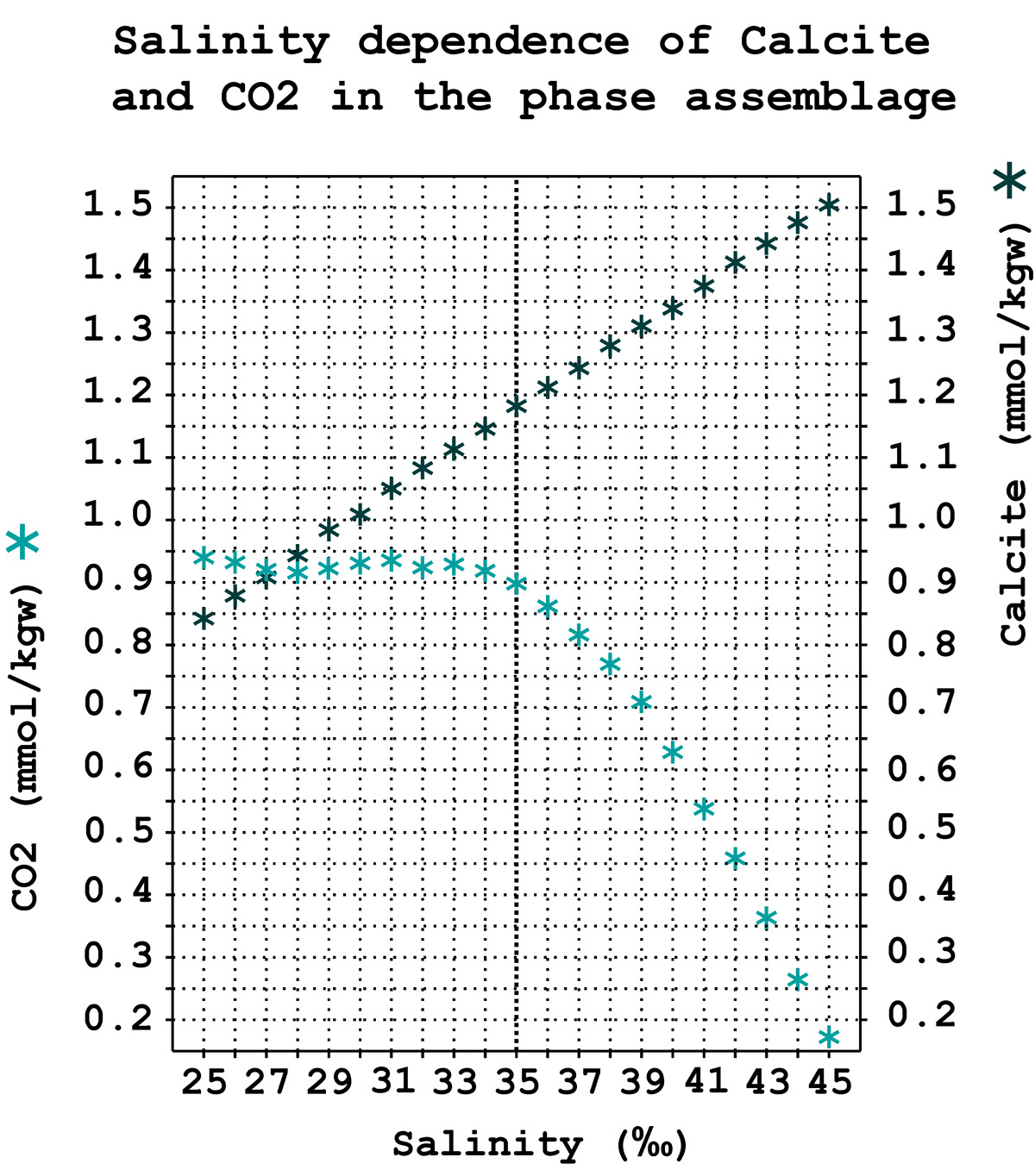
This linear dependence of salinity corresponds to a proportional increase in the thermodynamic potential, or Gibbs free energy, available to organisms to bind carbonate ions into crystalline minerals.
A small change in the salinity of seawater has a very significant effect on the deposition of carbonates. The average salinity of the ocean is 3.5 g/L or 35 ‰. And a small difference around this value between 3g/L and 4g/L for only 1 gram of salts per liter of water, ratio 1/1000, leads to a drastic change in carbonate deposits on the bottom: from 0 to 100%.
An example is shallow-water carbonate factories. For example, the Bahama Banks, where salinity increases due to evaporation and, in accordance with the laws of thermodynamics, aragonite, an unstable form of CaCO3, falls out of the solution, which then recrystallizes into calcite.

In the hot period, when the salinity of surface waters reaches 40-41 ‰, there is a "whitening" – direct spontaneous precipitation of mineral suspension, "lime powder" from the seawater. Studies show that precipitation is abiotic.
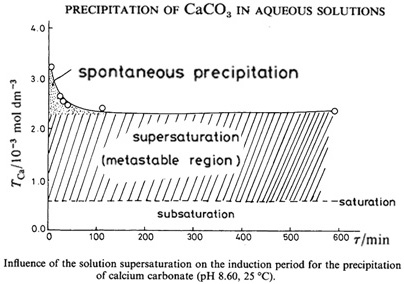
The same "whitening" is observed under similar conditions in other carbonate factories: the Red Sea, the Persian Gulf and other places with white beaches of CaCO3, the main carbon sequesters on Earth.
The productivity of these factories is very high. Thus, the average sediment of the Bahama Banks is about 600 grams of calcite per 1 square meter per year. Calculations show that such a carbon pump is able to suck out all the CO2 from the atmospheric column above it (400 grams) in less than 1 year.
In our research, we show that it is possible to create artificial carbonate factories that are not inferior to natural ones, and even surpass them. They can also be located in open areas of the ocean, provided that the depth does not exceed the carbonate compensation depth (CCD). The technology can be scaled, it can effectively sequester anthropogenic CO2 and stop Global warming.
 You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
Artificial increase in the salinity of surface waters, increased foraminiferous rain, or direct physico-chemical precipitation of carbonates has similarities with laboratory and industrial technologies of salting out - the separation of soluble (usually organic) substances from aqueous solutions by adding electrolyte solutions to them.
Salt-induced precipitation of both organic substances and inorganic minerals is the result of the same combination of factors: an increase in the ionic strength of the electrolyte and common‑ion effect.
The system of coupled elements of seawater has an electrical nature. The activity of individual ions and complexes depends on the Ionic strength, a quantity representing the strength of the electric field in the solution:
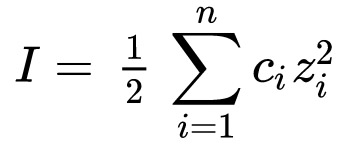
where I is equal to half the sum of the products of the molar concentration ci of each ion (M, mol/L) and the square of its charge zi. The sum is taken over all ions in the solution. Due to the square of zi, multivalent ions (Mg2+, Ca2+) contribute particularly strongly to the ionic strength.
Quadraticity in this equation follows from Coulomb's law, according to which the force of interaction of two point charges is proportional to their magnitudes and inversely proportional to the square of the distance between them. However, water molecules as a dielectric weaken the Coulomb interactions of ion pairs, triplets, and clouds. These ion clouds shield the charge of the central ion, which is the reason for introducing activity as an "effective concentration" in ions.
Typical values the Ionic strength:
potable and groundwater I = 0.001 – 0.02M,
seawater I = 0.67 – 0.71M .
 You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
To answer the question: How quantitatively does the salt content in seawater affect the deposition of carbonates? We created a model with major ions (99.9 %) in seawater: Na+, K+, Mg2+, Ca2+, Sr2+, Cl–, SO42–, H2BO3–, Br–, F –, HCO3–, CO32–, OH – and H +.
Simplified model of the interaction of major ions in seawater
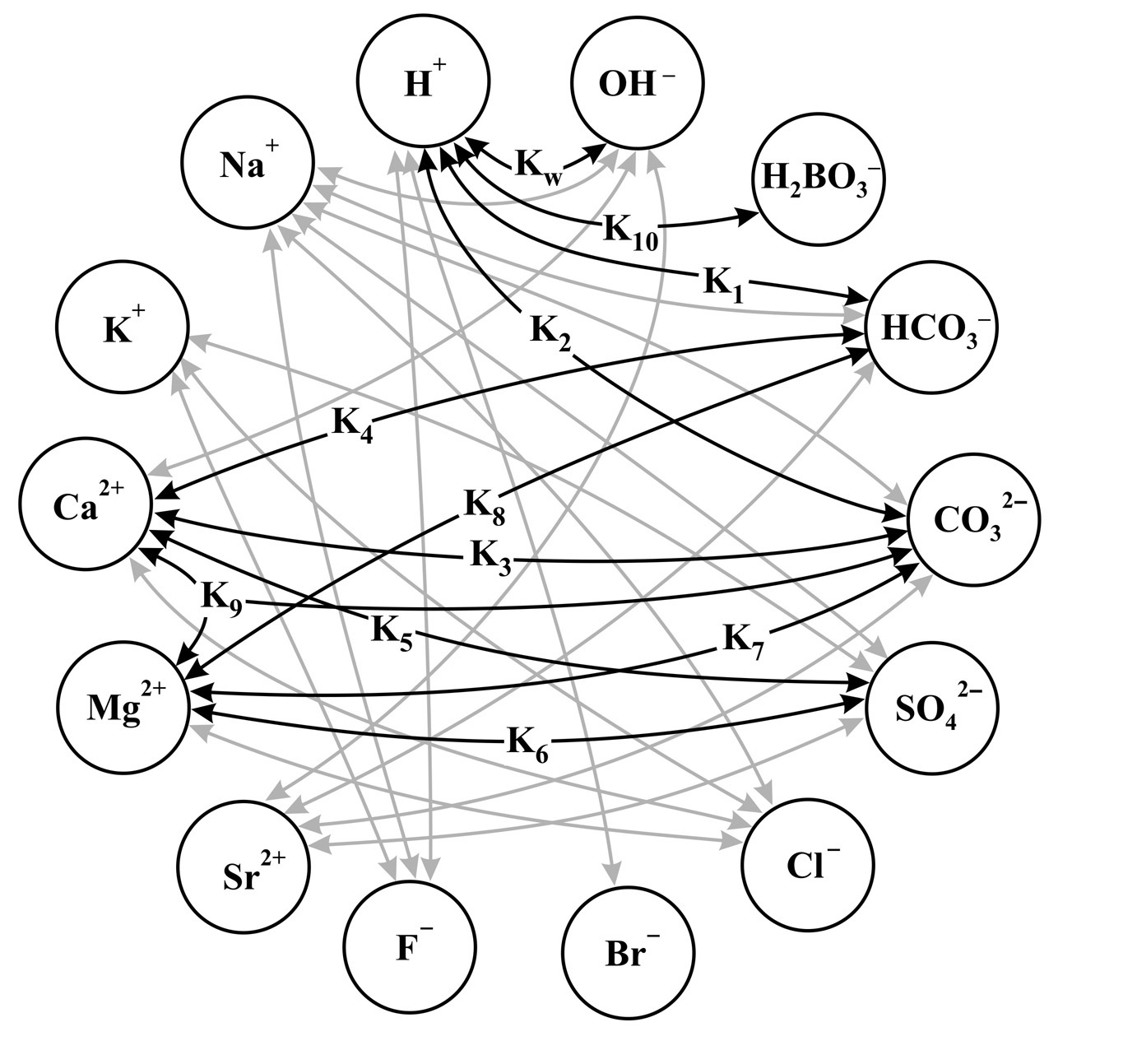
System of 65 equations with unknowns, 11 of them are key, the rest are less significant, however, neutral ions such as Na+, Cl– set significant contribution to the value of ionic strength due to their high concentration.
Heterogeneous equilibrium in seawater is described by a system of equations that are derived by substituting the equations for moles of species derived from mass-action equations into the equations of molar balance and charge balance.
In our calculations, we used a system of Pitzer equations, the parameters of which are linear combinations of the parameters of the virial expansion of the excess Gibbs free energy, which characterize the interactions between ions and a solvent.
Using thermodynamic databases for all seawater substances, numerical values of thermodynamic properties of individual substances such as entropy S (J/K×mol), enthalpy H (kJ/mol), heat capacity Cp (J/K×mol), Gibbs energy change ΔG (J/mol), and calculate log10(Kp) – decimal logarithm of the equilibrium constant K of the reaction of dissociation, it is possible to calculate the interactions of all ions in seawater and quantify the effect of salinity on solubility and precipitation of carbonate minerals.
If you like numbers like us, then please visit the full desktop version. There you will find information about calculation methods, software, etc. Here we will present only the results.
The quantitative indicator of calcite, CaCO3, which must be precipitated from seawater to achieve equilibrium, is the Calcium Сarbonate Precipitation Potential, CCPP. The equilibrium is termed a “phase assemblage”.
Chemical thermodynamics determines the changes in variables in the process and characterizing the state of the system. The word "time" is absent in this branch of physical chemistry.
The regularities of the course of chemical reactions over time are studied by Chemical kinetics which mathematically models reaction rates. In this case, we are dealing with a reversible reaction described by formula.
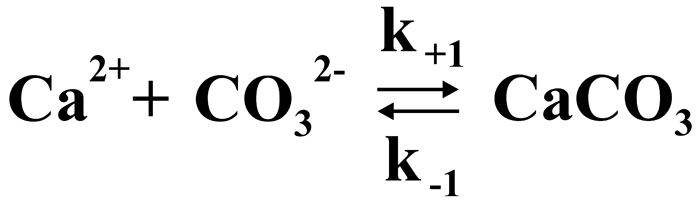
where k+1 and k–1 are the forward and reverse reaction rate constants.
The change in reaction rates is associated with a change in the concentrations of reagents and is expressed in general terms using the law of mass action for the reaction rate. The rate of a chemical reaction at each moment of time is proportional to the concentrations of reagents raised to degrees equal to their stoichiometric coefficients.
The point of contact of chemical kinetics and chemical thermodynamics is the equilibrium constant Keq
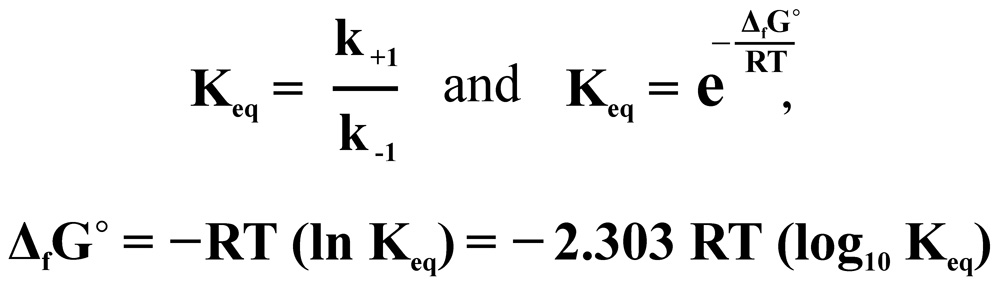
where ΔfG° is a Gibbs free energy change per mole of reaction for unmixed reactants and products at standard conditions, T, is an absolute temperature, R, is a gas constant.
We are working on creating a unified kinetic mathematical model based on rigid thermodynamics and including arrays of real data. This model should be global, scale freely in all directions, and consist of super- and hyper‑assemblages.
The main conclusion of the study is the paradoxical statement that sea salt is a catalyst for the precipitation of carbonate minerals. It not only significantly accelerates this process, but can also initiate a reaction.
The paradox lies in the fact that by influencing the reaction rate and time, the mechanism is fundamentally different from the classical scientific understanding of catalysis. In which, according to the theory of intermediate compounds, the catalyst repeatedly enters into an intermediate chemical interaction with the reaction participants and restores its chemical composition after each cycle of intermediate chemical interactions.
The main agents of salt catalysis, Na+ and Cl– ions, are neutral, do not enter into chemical reactions and do not form intermediates. The influence on the reaction time is carried out physically, by increasing the strength of the electric field of the solution - the ionic strength. Nevertheless, the results of catalysis are obvious: the rate of deposition of carbon in the form of carbonate sediments is directly proportional to salinity. The more salt there is in seawater, the more calcite there is at the bottom of the reservoir.
Limestone, chalk, marble and calcite white beaches are the result of the high salinity of the water. It is surprising that nobody understands, investigates, or uses this obvious fact in nature.
The second intermediate conclusion of the study is the statement that in the quantitative ratio of salt/calcite for the practical purposes of CO2 utilization, the key concept is time.
In the ''Bahamian model'' of an artificial carbonate factory, the salt/CO2 ratio is balanced as 1/1 in 34 years. That is, 1 ton of salt dissolved in the sea under certain conditions will lead to the absorption of 1 ton of CO2 from the atmosphere and deposition to the bottom in the form of CaCO3 in the specified time.
And the third conclusion, which few people know yet, is that there is
sea salt on land more than in the ocean.
In the west of the country there is a vast area around the Caspian Sea called the Caspian Depression or the Pricaspian Lowland, the level of which is below the level of the Ocean.
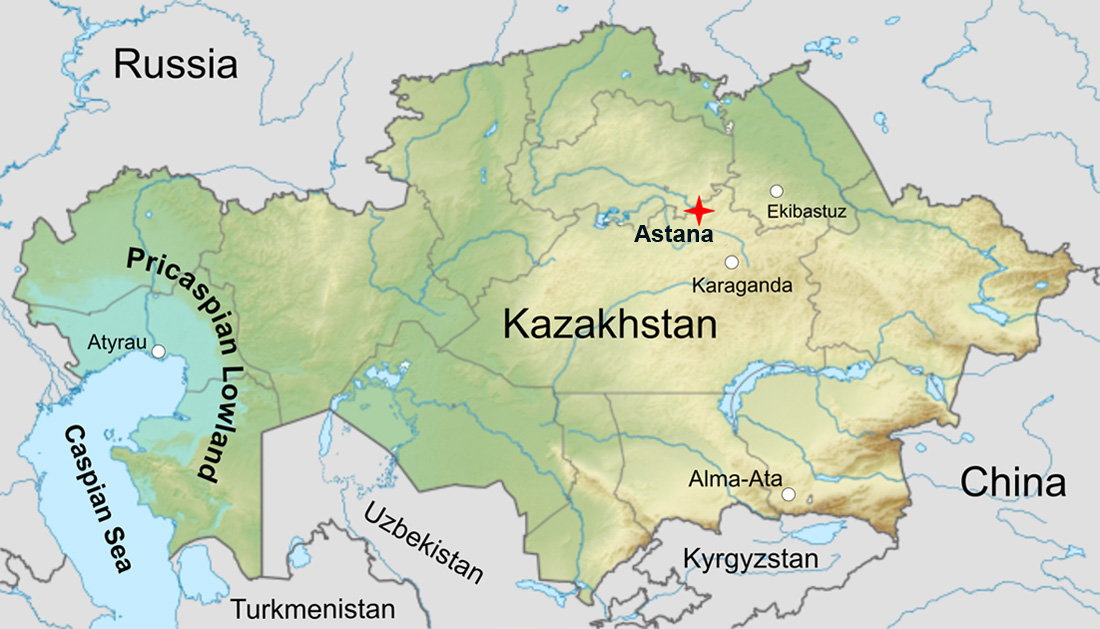
There is the world's biggest evaporite deposit of salt of the ancient ocean. Gigantic volume of 1,6 mln km3 rock salt is 20 times greater than the volume of the all Caspian seawater (78 000 km3) or about 3,5 mln gigatons (petatons), is as much salt as evaporated from about 1/10 of the World Ocean.
Salt lies here in an almost continuous layer 2 – 5 km thick, dotted with salt formations called salt domes, as well as pillars, mushrooms, walls, diapirs, with a depth of 8 – 10 kilometers and diametrical dimensions from several to several hundred kilometers. Salt domes grow 0,5 – 1,3 mm per year from the ancient Permian layer about 300 millions years old, and most of them have reached the surface, on which, as a result of metamorphic weathering and leaching, stone hats or "caprocks" have formed. They composed of gypsum (CaSO4:2H2O) and fragments of poorly soluble rocks. Caprocks are usually 10 – 20 meters thick and look like small hills. In a number of domes, salt comes to the day surface.
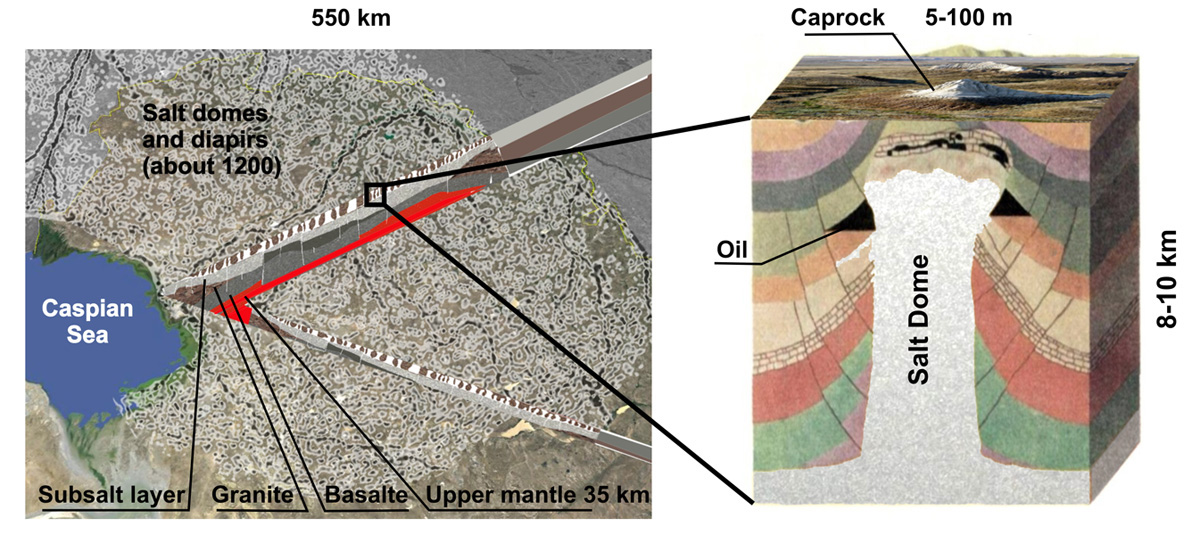
Some of the domes are gigantic. For example, the area of the Shalkar salt massif is 2700 km2. It was formed by the confluence of at least seven separate stocks and contains 25,000 km3 of salt. Other large domes are Satimola and Inder with an area of 250 km2.
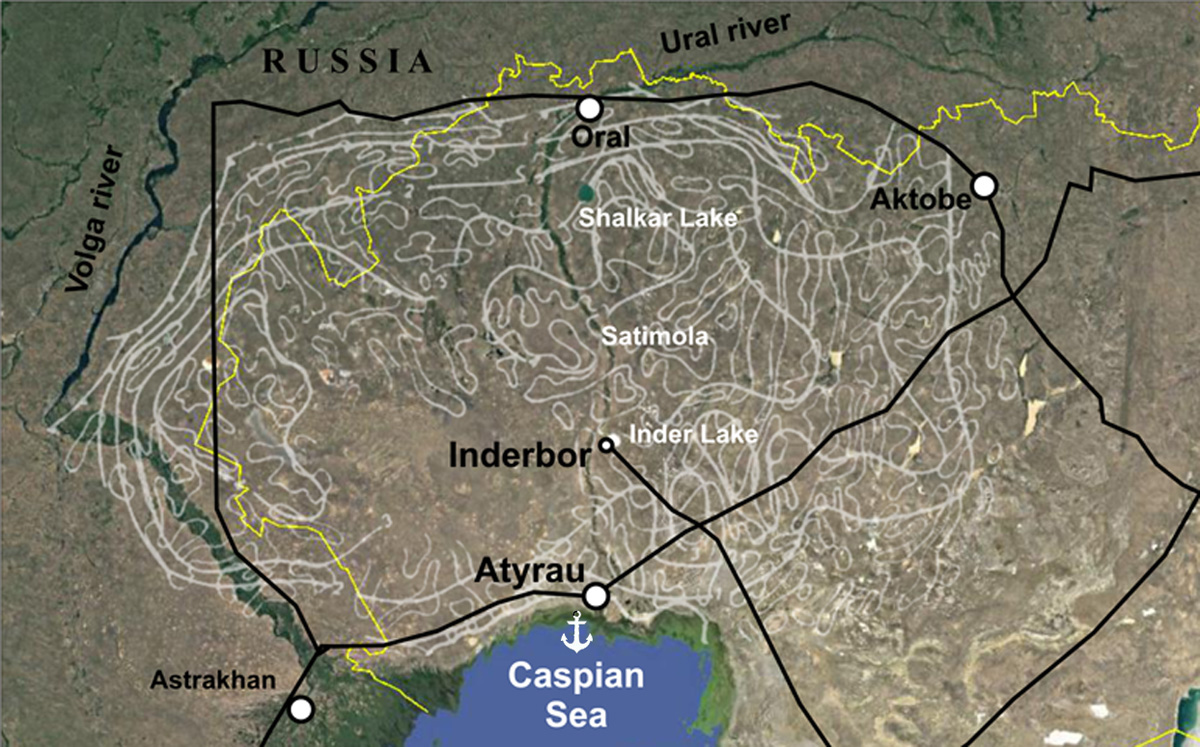
On the map, light lines represent the boundaries of salt accumulations, black lines are railways. Salt domes emerging to the surface often have a flat dome mirror due to surface metamorphism. They represent uplifts in the form of a plateau-like hill, rising 20-25 meters above the surrounding desert.
Due to the different nature of the dissolution and precipitation of salts, there are numerous deposits of potassium salts and borates. One of these is a large borates deposit in the area of the salt lake Inder, 150 km north of Atyrau. The Inder borates deposit has been industrially exploited since the 70s, and intensive opencast mining began in the 80s. However, after Kazakhstan gained its independence, the production and enrichment of borate raw materials stopped. Currently, the town of Inderbor specializes in the processing of building gypsum, which line the entire plateau with a thickness of about 50 meters.


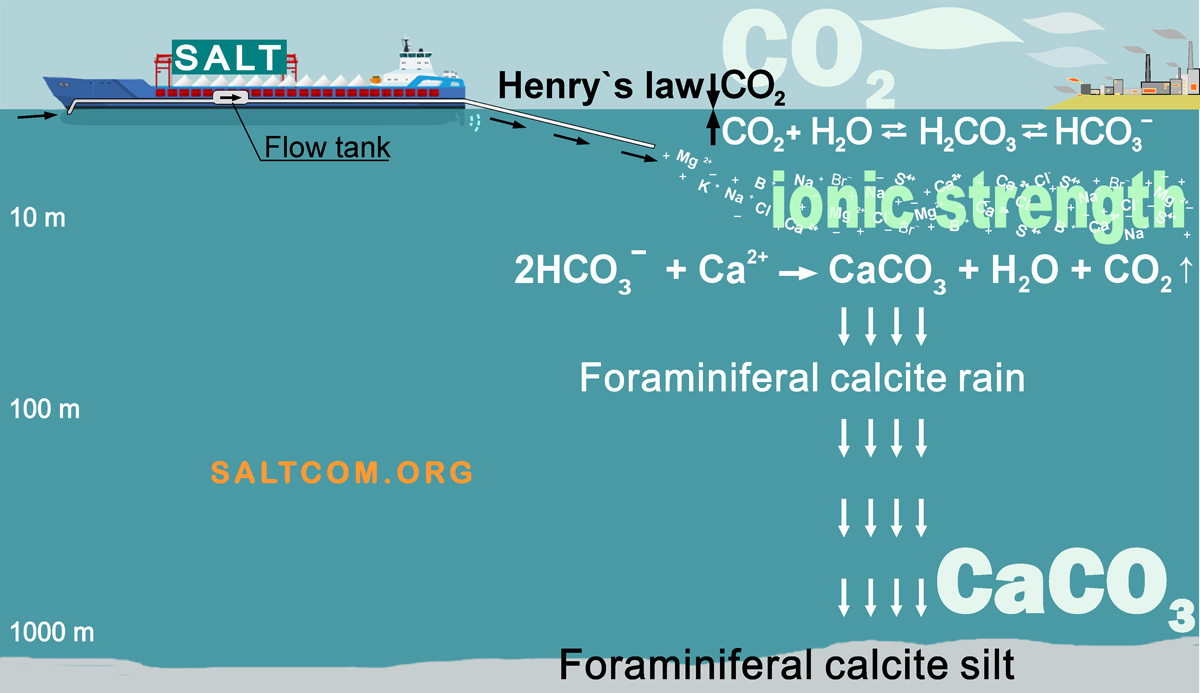
Of course, salt should be added to seawater only in dissolved form. So that the local concentration does not exceed the total salinity by more than 4-5‰. This can be done using a flow tank, which is found on almost every ship, and also pumps, valves, sensors for water flow, salinity or an measuring instrument thermosalinograph.
To accelerate the dissolution of salt, it can use air bubbling from the compressor. Double-circuit circulation is also possible.
Since salting is limited in concentration, it is reasonable to expand it horizontally in order to increase productivity using ship-harvesters or carbon removers connected by a brine pipeline.
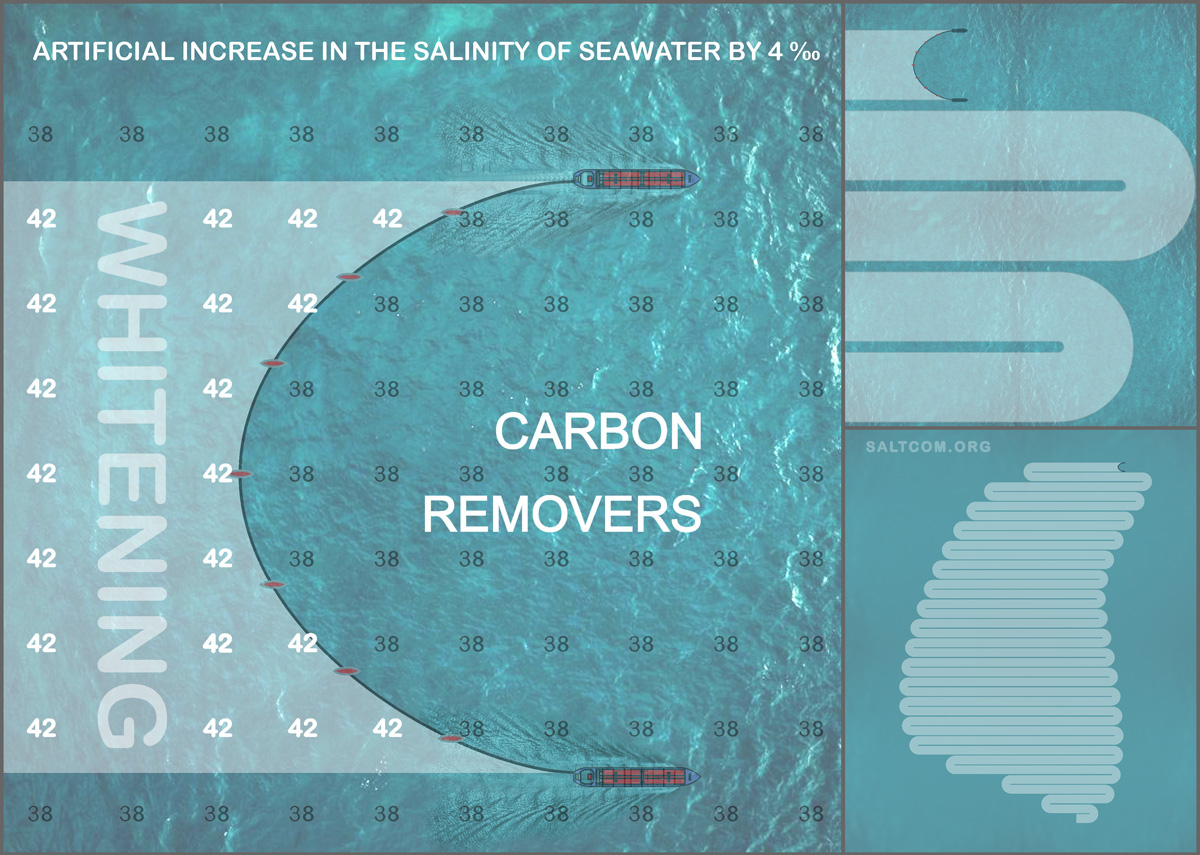
With help of these carbon removers, we can draw additional lungs for the planet.
Speaking in sports, a second breath will open.
This mild, environmentally friendly impact on the carbon balance system will work as a CO2 pump from the atmosphere. The widespread adoption of technology will have a noticeable, experimentally measurable effect. And since there is no limit to scaling, it will evolve into a giant climate industry.
Together with international efforts to reduce CO2 emissions, its active sequestration in the depths of the Ocean will first slow down global warming and then completely stop it. And even be able to reduce atmospheric CO2 from today's 417 to 280 ppm pre-industrial levels in 1850. Back to the future.
In the future, humanity will be able to control not only the global climate and atmosphere, but also control the weather, influence it, for example, cancel hurricanes (see below Vision).
But already now it is possible to control the change in the salinity of the Ocean's surface from space using a satellite
Soil Moisture and Ocean Salinity (SMOS) of the European Space Agency.

The spacecraft has been scanning the planet's surface in the microwave range for 12 years and generating a surface salinity map with an accuracy of 1‰. The interesting Big Data has been accumulated and available to everyone on the mission website.
![]()
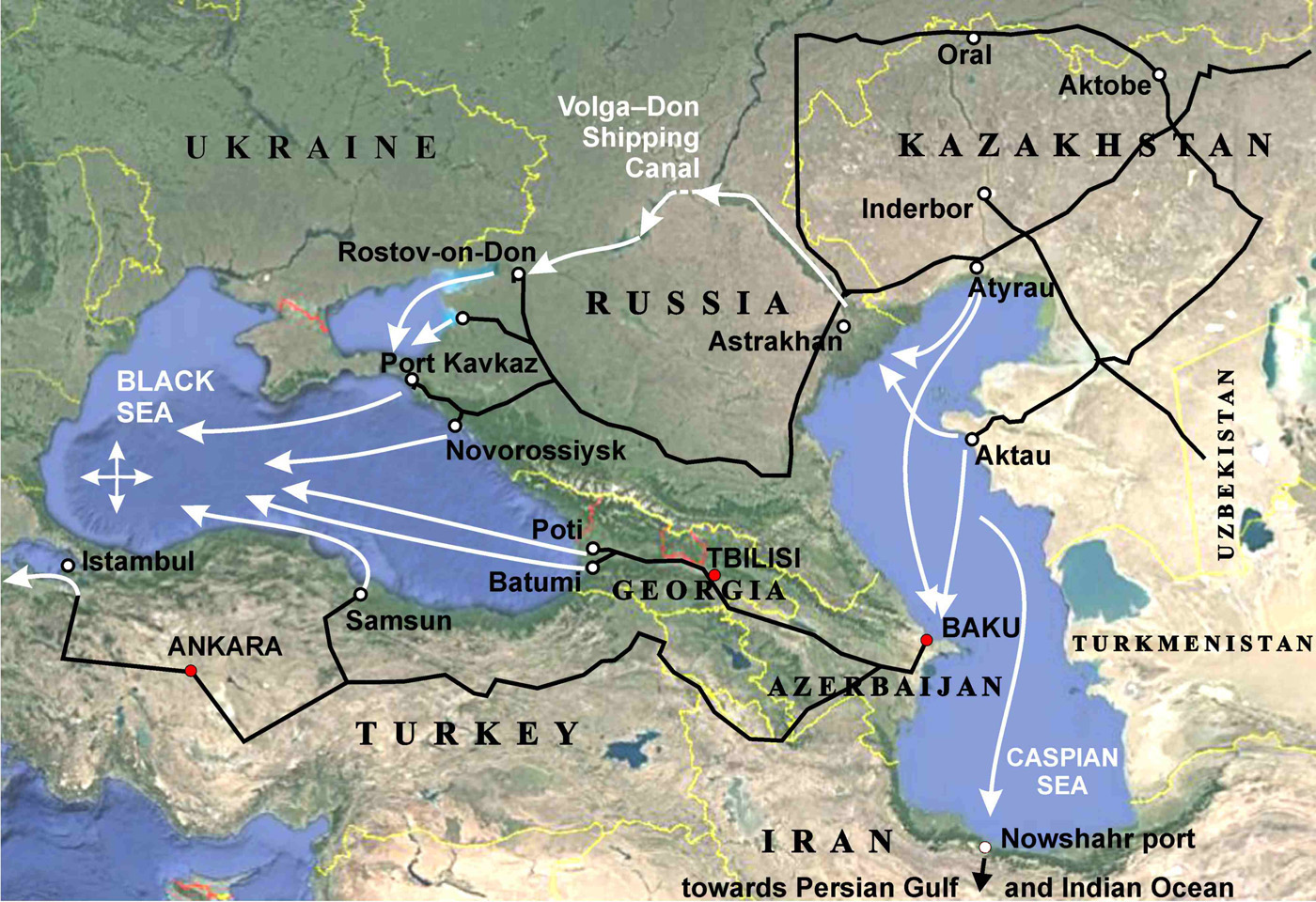
Salt deposits are found on all continents of the Earth, in many countries and under the seabed. Salt is extracted by open pit mining, underground mine workings and in liquid form by drilling wells, pumping out and evaporating brine. In places where the transport leverage of the logistics allows for the transport of brine water, a liquid CO2 sequestration scheme is possible. In addition to brine mining, Underground Gas Storage is also widespread in the form of artificially created caverns in salt layers. There are hundreds of such storage facilities for gas and oil products in a number of European countries, USA, Russia and China. Technologies for the creation and operation of underground reservoirs have long been developed.
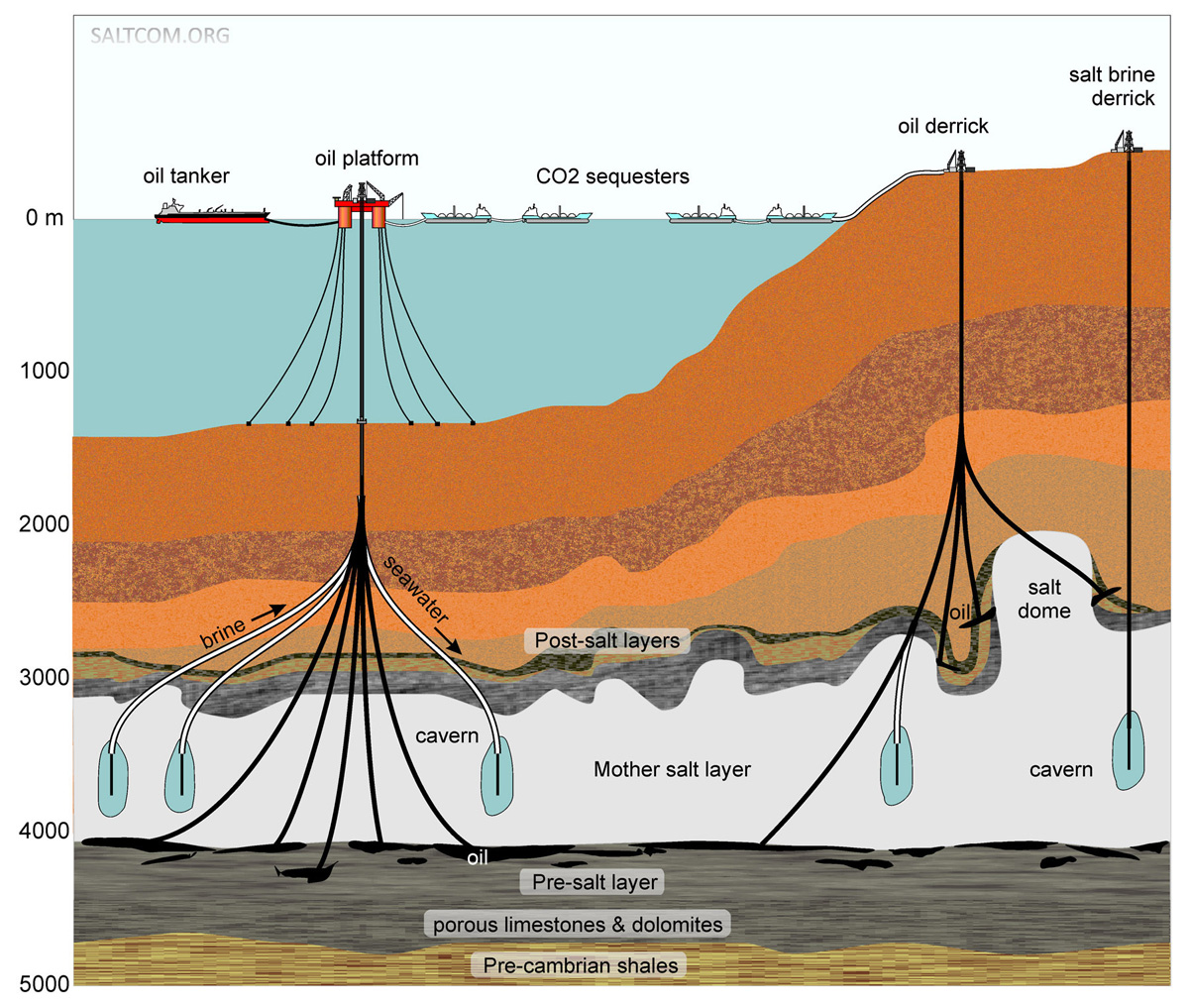
The mother salt layer is of varying thickness. In the salt basin of the Gulf of Mexico, it averages 1–1.5 km, under the bottom of the Persian Gulf, about 2 km, under the bottom of the Atlantic in the Santo salt and oil basin near Rio de Janeiro 3–4 km, and under the northern Caspian Sea 4–5 kilometers, much more than a layer of seawater above it, 5–25 meters.
The world's total land and underground salt deposits (not dissolved in seawater) are very large. Currently, nobody has yet quantified their mass. Based on the study of a large amount of scattered data about all salt deposits of the Earth, it became clear that in the land and underground salt is more than in the Ocean. We estimate it by 1.5 times or about 60 mln gigatons. About the equal as estimated total sedimentary carbonates (> 60 mln Gt) in the form of limestone and dolomite.
![]()
Everyone knows the roof of the world, the Himalayas and the highest mountain of the Earth, Everest, 8.85 km high, but few people know that in the Caspian Lowland there are dozens of underground salt mountains with a height of more than 10.7 km.
We open the salt of the Earth for the society.
![]()
Surprisingly, in the 90-year history of the idea of ocean fertilization (fertilizing with iron since 1930), there is not a single proposal to look at the problem comprehensively.
Everybody is looking for "magic pills" like iron, nitrogen and phosphorus or some cocktails of elements, but nobody has seen the entire table of elements dissolved in water in the form of sea salt.
Everybody worries that glaciers and polar caps are melting, but nobody has thought about where this melt water is going (spreads over the ocean surface due to the fact that fresh water is lighter than salty) and how to compensate for this desalination or acidification of the ocean, "evil twin of global warming".
Everybody worries about the death of corals. They even found out the salinity limit for their vital activity (>32 ‰). But nobody said:
SALT WATER! HELP CORALS!
We have studied thousands of scientific papers and documents, but nowhere have we found such a simple idea. In the scientific world, it is completely absent. Maybe someone will say: I said before... in the kitchen. Well... We will say: very nice! Let's join us. We stay on the threshold of great change. Like a chinese wisdom says, the way of a thousand gigatons starts with one ton.
![]()
If we look at past changes in CO2 in the earth's atmosphere, we can see regular fluctuations in the 200 - 300 ppm range. Any scientist or engineer will confirm that in order to establish an oscillatory process, different direction forces with nonlinear feedback are needed. This is how all oscillators work. For example, an electric oscillator uses a cyclic transfer of energy between a magnetic field in a coil and an electric field in a capacitor. But what forces are involved in natural oscillatory cycles? If we exclude cosmological factors and temperature, which shows the thermodynamic result of these fluctuations, then there will be a carbonаte balance and a nonlinearly related ionic level of seawater or its salinity. Primarily its surface waters.
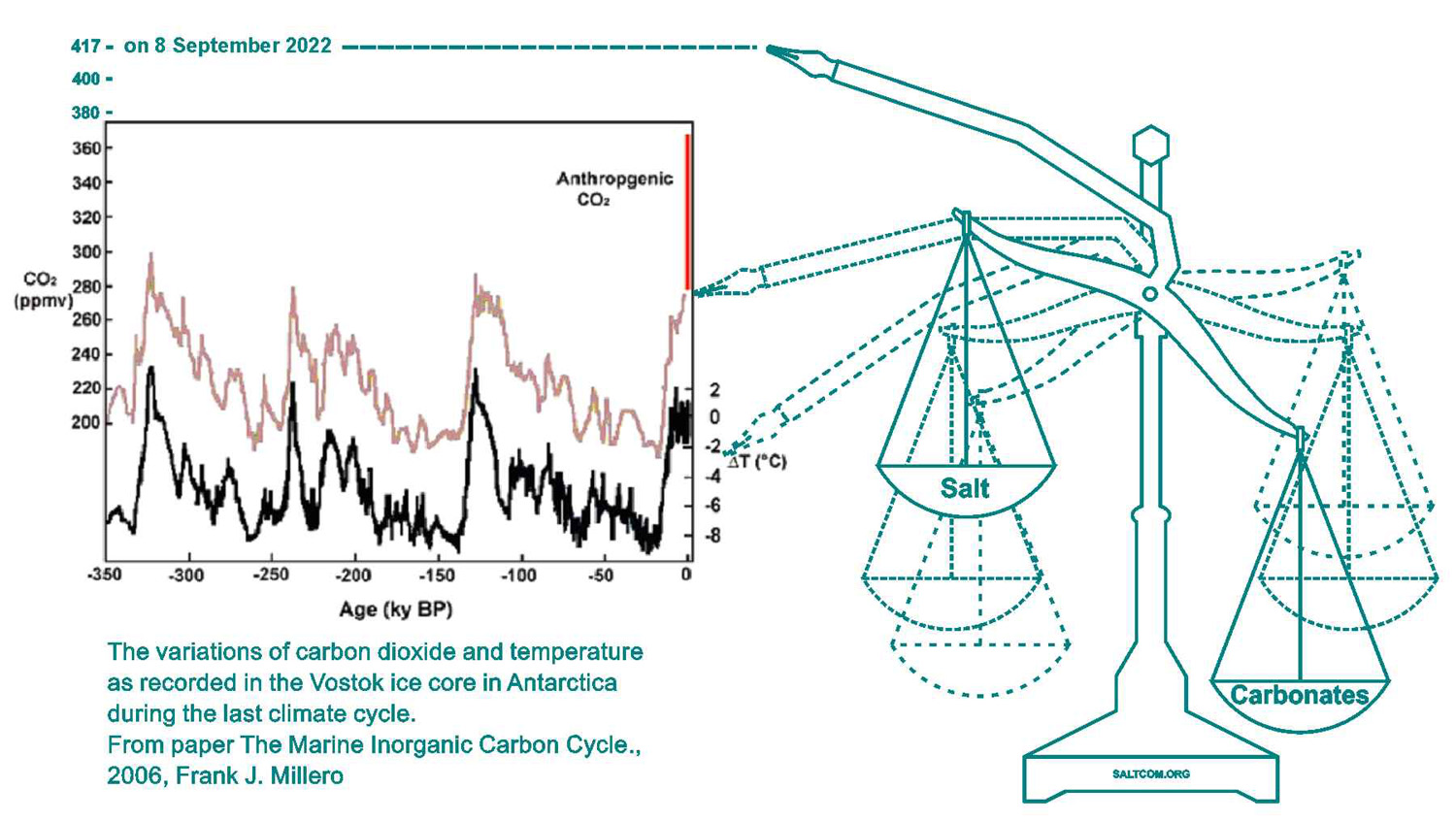
There is also the “coral reef hypothesis”, which states that shelf flooding during glacial melt and ocean level rise is a significant contributor to carbonate deposition and therefore affects atmospheric CO2 levels. This hypothesis does not contradict ours, it can be considered as subharmonics of the general oscillatory process in nature. Since carbonates are salts of carbonic acid, this is the flow of energy between carbon and non-carbon salts.
Coral polyps contribute to the deposition of carbonates. However, it is significantly less than the total contribution of calcite-fixing unicellular microorganisms, such as foraminifera and coccolithophorids. In addition, these microorganisms are present everywhere, not only in shallow water, but also in deep waters and on the seabed.
Among the three main factors of carbon balance in the ocean: temperature, pressure and salinity, at low values, pressure has the smallest effect. Lysocline, the boundary of dissolution of carbonate deposits is located at a depth of several thousand meters and at a pressure of hundreds of atmospheres. 10 meters of water or 1 atm is very small values. Whereas small changes in salinity of 1 ‰ significantly affect the deposition of carbonates from seawater and the absorption of CO2 from the atmosphere.
![]()
At the level of everyday understanding, salt is an antiseptic and a conservant. Since ancient times, its ability to suppress the vital activity of microorganisms has been known. Here it is appropriate to say in the words of Paracelsus that everything is poison, everything is medicament, and both are determined by the dose ("The dose makes the poison").
Nobody from readers of this document will be able to taste the difference in salinity of water 2-4 ‰. Perhaps dolphins or whales will be able to, but this will not affect their life in any way, because they make regular migrations from the surface to a depth of tens and hundreds of meters, and some even kilometers, constantly living in the same conditions of changing salinity. If marine animals live in the coastal zone, then they experience much greater differences in salinity, up to 10 ‰ and higher.
The vast majority of marine life can be considered euryhaline organisms for such small differences in salinity. Mollusks, jellyfishes, arthropods, sea urchins, worms, turtles and all kinds of fish. Many common species such as herring, sprat, sea bass, sharks and others easily tolerate changes in salinity of more than 20 ‰, and species such as, for example, salmon and eel can generally live and spawn in completely different waters of the sea and rivers with a salinity difference from 0.3 to 38 ‰.
Microworld of a simplest unicellular organisms demonstrates a high tolerance to small changes in salinity. Photosynthetic cyanobacteria, including the smallest piko-sized species Prochlorococcus and Sinecoccus, which absorb more CO2 than all of Earth's forests, also have a large salinity gap. Cyanobacteria, as the most ancient species, gravitate towards life in conditions of increased salinity than today. There is some utility in the increased ionic level of seawater.
This is noticeable in comparison of salty seas such as the Red or Caribbean, where we observe an abundance of life with the less salty Baltic and Caspian seas with relatively scarce flora and fauna.
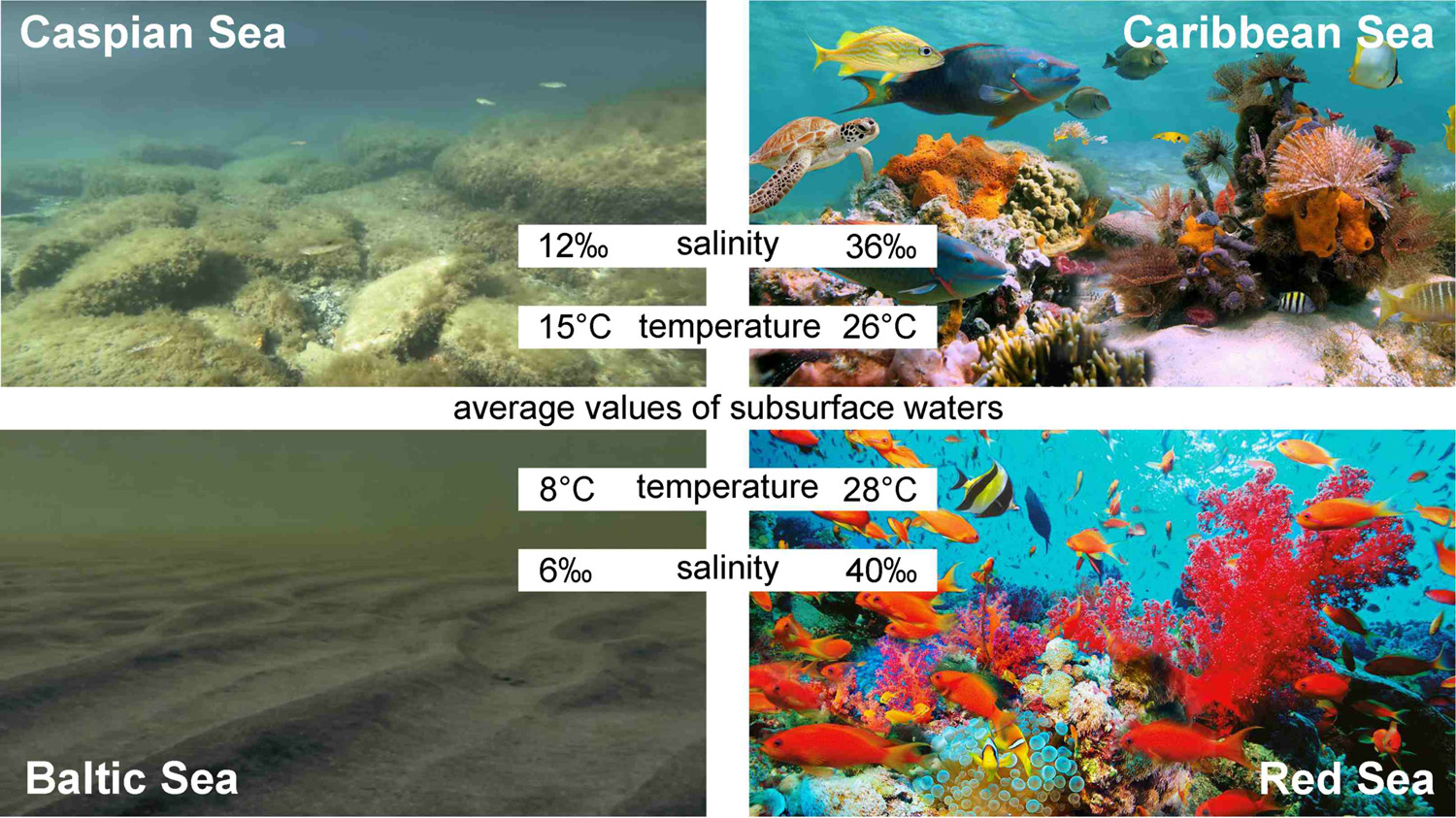
We do not agree with the stereotyped cliché that forests are the lungs of the planet. The absorption of carbon dioxide by forest vegetation is compensated by the decomposition of dead organic matter back into water and CO2. Since the Carboniferous period (360-300 million years ago), many aerobic and anaerobic bacteria and fungi have learned to effectively decompose cellulose, the most common organic compound on Earth (about 59 % of organic matter).
Any significant accumulation of carbon in the soil is impossible in modern conditions, most of it is returned to circulation. If this were not so, then we would walk at kilometer-depth layers of soil and organic humus, but in reality the average thickness of the soil is 20-30cm, and under the densest jungle does not exceed 2-3 m.
This means that O2 and microorganisms that decompose dead organic matter work faster than its photosynthetic producers. Therefore, planting trees as a way to sequester CO2 has a short-term effect. After the death of a plant, almost all of the stored carbon is released back into the atmosphere within a few years.
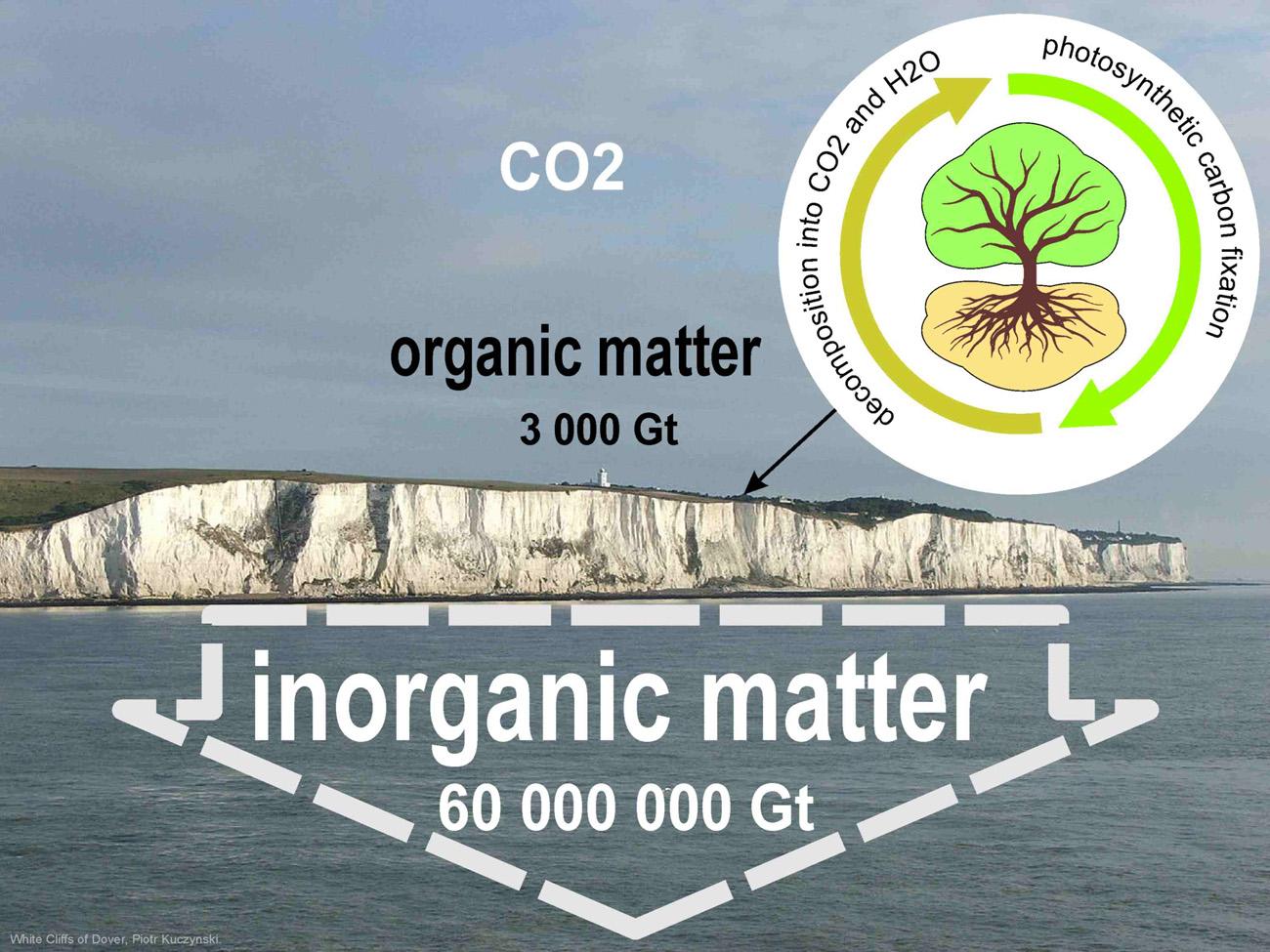
The real lungs of the planet are the carbonate factories of the ocean. For example, the Bahama Banks are a carbonate platform 4-8 km deep. This is the result of CO2 sequestration over the past 150 million years.
The amount of accumulated inorganic carbon on Earth is four orders of magnitude greater than the amount of all organic matter. Deposits of carbonates of kilometer and many hundred meters thickness are found everywhere. Examples are the Dolomitic Alps in Italy or the limestones of Europe's highest mountain, France's Mont Blanc. The ratio of organic-inorganic carbon is colorfully illustrated by the Chalk cliffs of the coast of the English Dover, about 120 meters high (together with subsurface layers of about 400 meters of carbonates).
In the ocean, the cycle of organic carbon is similar to land. A huge number of microorganism species decompose organic matter in the entire water column and up to several meters deep in bottom sediments. Remineralization or transformation of organic matter into its simplest inorganic forms is carried out through the electron acceptor cascade:
O2 reduction → NO3- denitrification → Mg2+ reduction → Fe3+ reduction → SO2- sulfate reduction → CH4 methanogenesis.
The cycle of organics is closed so that only 1 mole out of 1000 has a chance to be deposited in kerogen for a long period of time. Comparing organic and inorganic carbon dioxide sequestration as machines with 0.1 and 100 % efficiency is hardly relevant.
![]()
Since CO2 storage is effective in inorganic form only, we do not want us to be confused with various "fertilizers" of the ocean and other accelerators of organic growth. We have nothing to do with pseudoscientific tricks, do not replace reality, but look into the essence of nature through the laws of thermodynamics. The calculation of all changes in Gibbs energy indicates that our technology is perhaps the only energetically profitable today. In any case, among all globally scalable.
The introduction of individual elements into seawater is an imbalance that leads to a chain of effects that does not have long-term results in carbon storage, and often negatively affects natural CO2 absorption. For example, dissolved phosphates are the main inhibitory factor for CaCO3 deposition in seawater. That is, both intentional "fertilization" and careless runoff of agricultural fertilizers through rivers into the ocean is a direct braking of the natural process of carbon storage.
The main claim of ecologists to the fertilization of the ocean with iron salts, nitrates, phosphorus compounds, etc. is a concept of "pollution" – "this is the introduction into the natural environment or the emergence in it of new, usually not typical physical, chemical or biological agents (pollutants), or the excess of their natural average long-term level in various environments, leading to negative impacts".

The fish kill due to an imbalance of ions and the rapid reproduction of microorganisms during the harmful algal bloom.
But sea salt is not a "non-typical agent" for the sea. It is an integral part of seawater, changing in local places in the ocean and at different depths (halocline). All insoluble salt on the Earth is the result of the evaporation of the oceans, in which the ratio of ions has not changed significantly over the past 500 million years. Of course, various salt deposits can have a variable composition depending on the conditions of deposition, but the advantage of salt, as mineral raw materials, in its simple averaging to sea composition both in dry form and through the brine.
By increasing salinity, on the contrary, we are helping the ocean to work better, absorb CO2 pollutant emissions. We strengthen its stability, increase the buffer capacity. There are no negative impacts. We only return the accumulated to nature, restoring the balance. We are driving the overall growth of ocean biomass. Everything becomes proportionally larger. More picoplankton, more plankton, more fish, more dolphins and whales. Ecologists have nothing to blame us for.
Let's say even more. More energy. More cement and steel, houses and factories. More cows and more food. More people,
![]()
We are opposed to the modern philosophy of "anti-growth" and are skeptical about the contractual capacity of CO2 restrictions and effectiveness containment.
If you need to burn fuel – burn it, get energy, develop, emit CO2, but compensate for it. Take into account your unaccounted externalities. Please clean up after yourself.
Since the salt technology of CO2 removal is energetically profitable, and its cost is an order of magnitude less than production and burning of oil, salt can be built into the modern energy economy.
![]()
The deposition of insoluble carbonates and their deposition in the long term for thousands and millions of years can occur only in places with depths above the carbonate lysocline, at least 3 km. These are mainly shallow waters and oceanic continental shelves. Shallow waters located at a distance of 370 km from the baseline of the coast are the exclusive economic zones of coastal States.
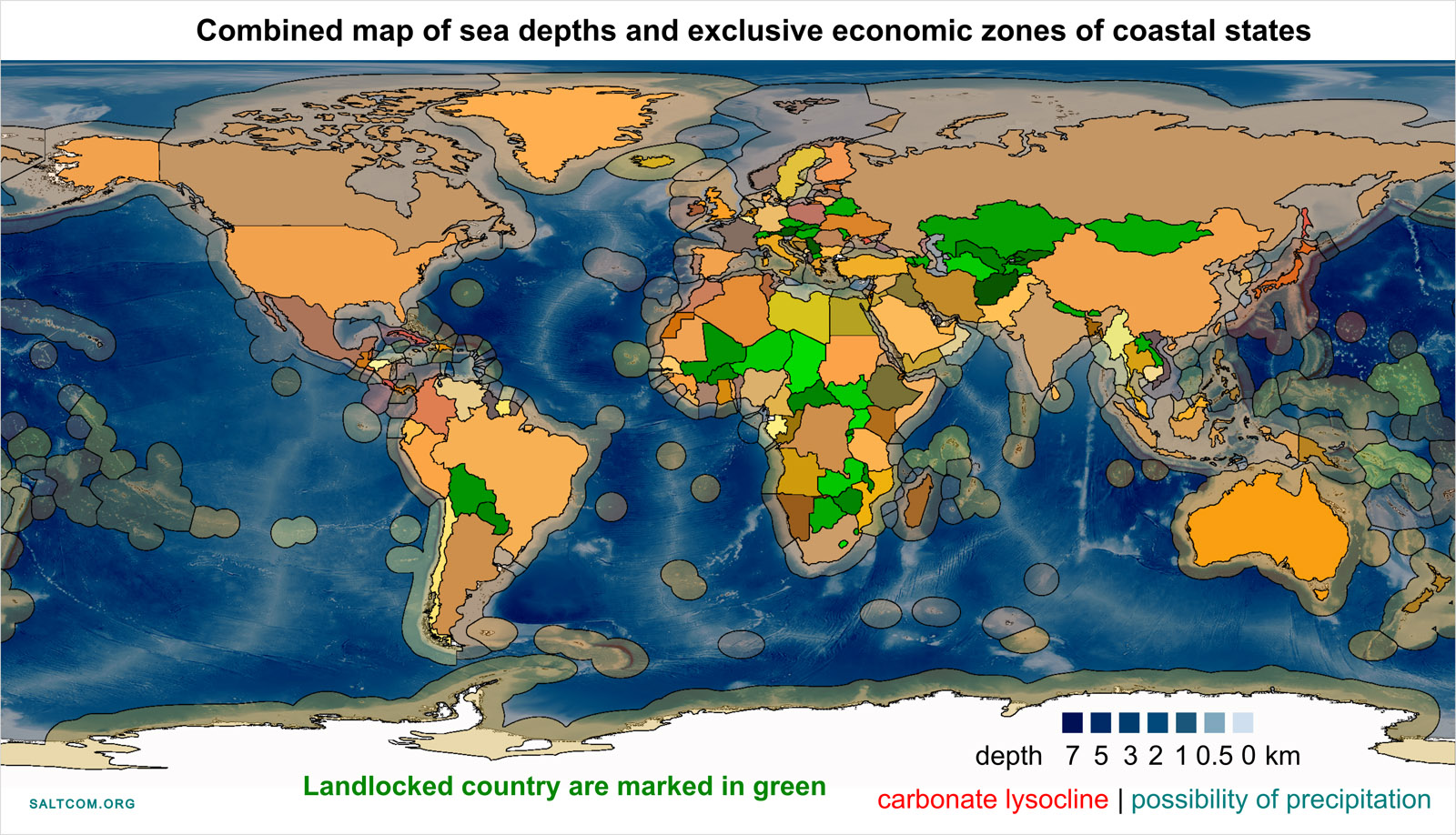
Many coastal countries will find themselves owners of natural carbonate factories that can not only clean the atmosphere from CO2, but also bring significant income to these states. Since the majority of the world's population is concentrated in coastal areas, and salt deposits are present in most countries, the obvious can be argued: countering the upcoming climate crisis is beneficial to everyone, and most have the potential to make money from it.
This climate technology will create millions of jobs and infrastructure for land and sea transport. It will become the driving force of global economic growth.
Seawaters suitable for depth and other characteristics also exist outside the exclusive economic zones in International waters, which are in common and equal use by all countries, including 44 landlocked country. Among which 15 countries have salt deposits and the largest is the Republic of Kazakhstan.
The atmosphere is the most variable environment on the planet. Its changes are largely related to changes in the ocean. It is thermodynamically coupled to water not only through Henry's law, but also through Raoult's law (both of these laws are interrelated), which relates the composition of water, its salinity, to the saturation vapor pressure above it.
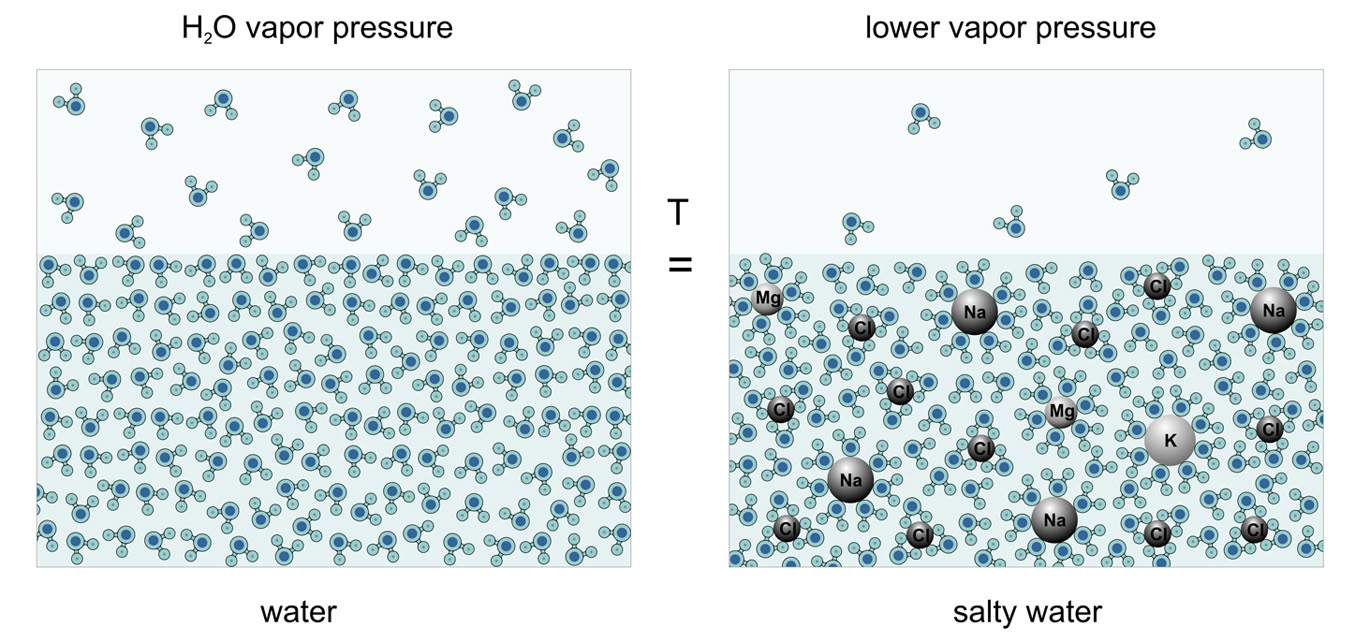
By changing the salinity of the surface, we can influence not only the absorption of CO2, but also the evaporation of water in the local place, thereby affecting its main atmospheric mover – water vapor, gaseous H2O. For given small changes in salinity of 2-4 ‰, the change in saturation vapor pressure is very small, but given the high volatility of the atmosphere, it can be used to influence air currents. Smart use of this instrument will allow, through small costs, to control the large forces and energies of nature.
The illustration shows how we will be able to deflect and cancel a hurricane.
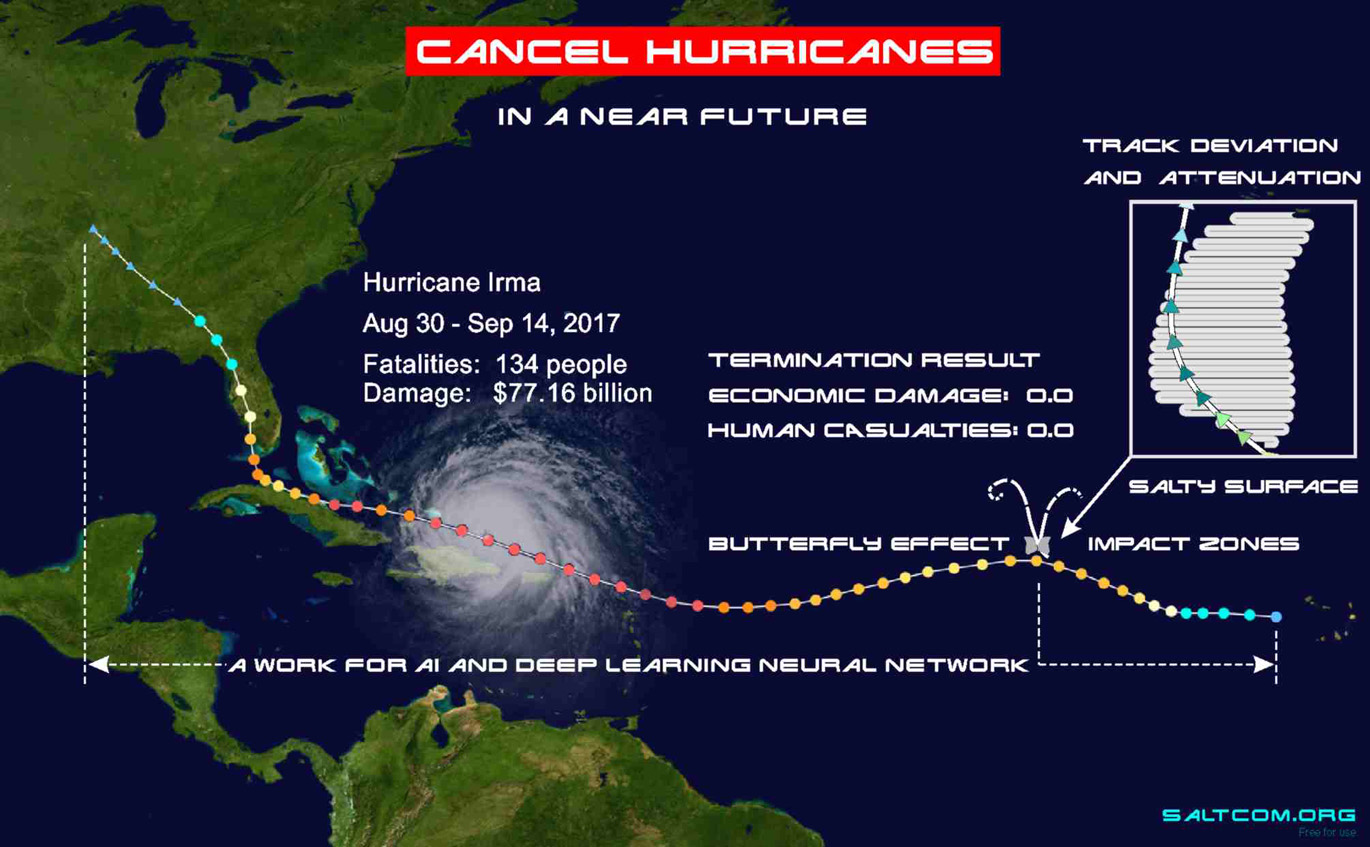
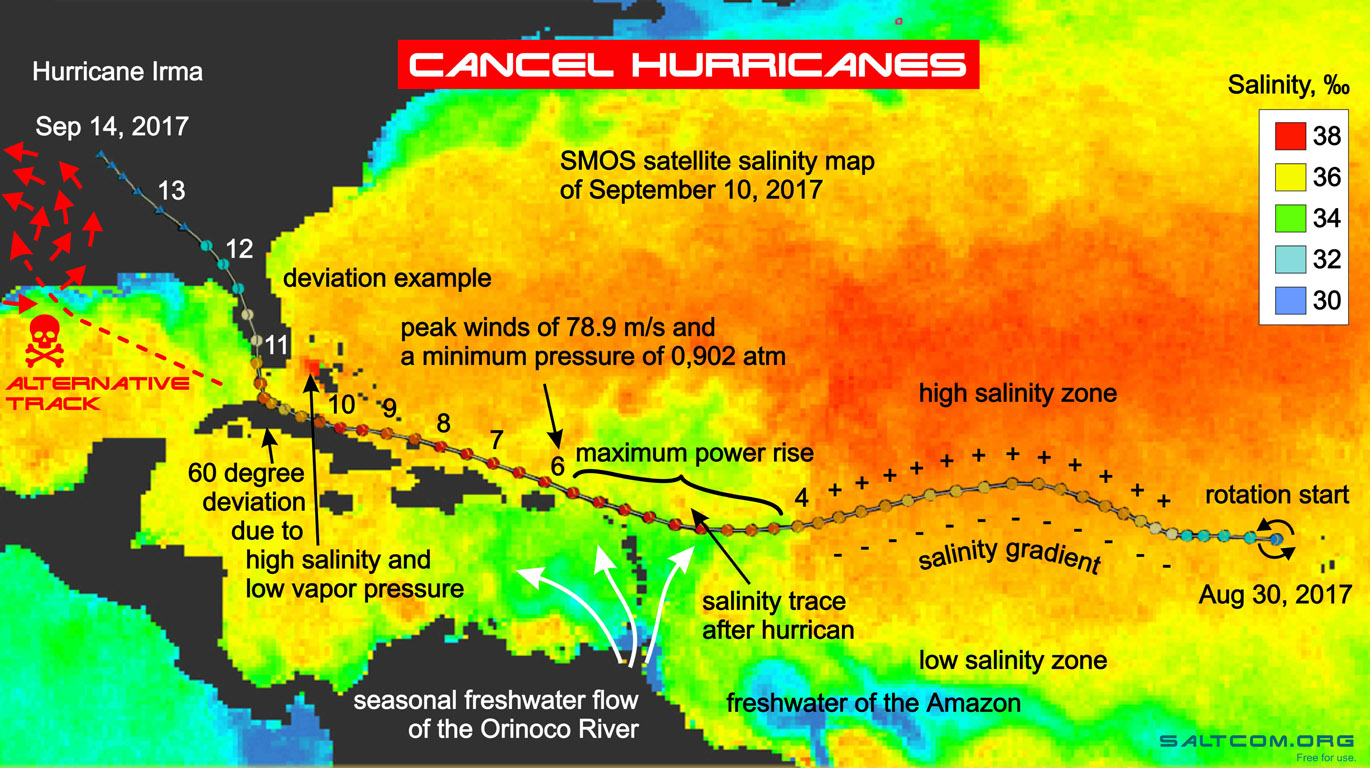
To see the future, we need to look mindfully at the past. In the illustration there is a map of ocean salinity, a surface scan from the SMOS spacecraft for September 10, 2017. We can see the timeline of evolution of hurricane and the factors that influenced it.
Irma is a devastating Category 5 Cape Verde-type hurricane associated with the name of the islands off the west coast of Africa. These hurricanes are powerful, usually appearing in the area of tropical waves that form in the African savannah during the rainy season. Crossing the Atlantic Ocean, they accumulate great energy and bring it down on America.
Passing along the path at relatively equal temperatures (the Sun evenly heats the surface of the Earth), the increase in their intensity is largely due to differences in the salinity of seawater. Above desalinated waters at the same temperature, according to Raoult's law, the value of the partial pressure of water vapor is higher, the internal energy of the system is greater. Therefore, there is a sharp increase in the category of hurricane over the flow areas of the large rivers of South America. On the map, we see how the hurricane reaches its maximum as it passes over the Orinoco River runoff area.
Here it should be noted that not only global warming, but also, to a large degree, the desalination of the surface waters of the World Ocean due to the melting of the polar caps and glaciers create a tendency to increase the frequency of natural disasters and the growth of their destructive power.
Local changes in salinity along the path of a hurricane can significantly change its trajectory. Possessing a large internal energy of water vapor, but a relatively small mass of moist air, a hurricane is very volatile in motion. Small areas of low or high salinity seawater can dramatically change the direction of its movement. We see how low vapor pressure over the salty waters of the Bahama Bank (red spot on the map) deflects hurricane's path 60 degrees towards Florida. If this salinity spot had not existed, then Irma have continued its way, increased its power due to the runoff of the Mississippi, and would have hit the western coast of the Gulf of Mexico. It can be said that the salty waters of the Bahama Bank harmed Florida, but saved Houston, Austin and Boca Chica from the superstorm.
If we take into account that the size of this salinity spot is much smaller than the hurricane (about 1/6 of its diameter), then it can be assumed that in the initial conditions of its formation, when the whirlwind was small, the artificial deflection area can be very small, only a few kilometers.
We are not going to chase tropical cyclones like hurricane hunters. Based on the accumulated data and numerical weather prediction, we get the opportunity not only to predict, but also to form events, change the weather and ultimately control it.
The answer to the intermediate question of life, death, home Earth, and everything, may be the Great American Saltwall — a set of the artificial carbonate factories which may be located above the Mid-Atlantic Ridge. There are suitable conditions for the precipitation of carbonates: a depth of 1-1.5 km, high temperature and salinity. The bottom sediments here are half carbonates (40-60 % CaCO3, see the carbonate sediment map↑).
The map shows the tracks of 19 devastating Category 4-5 Cape Verde-type hurricanes that killed more than 4000 people and caused more than $300 billion in cumulative damage.
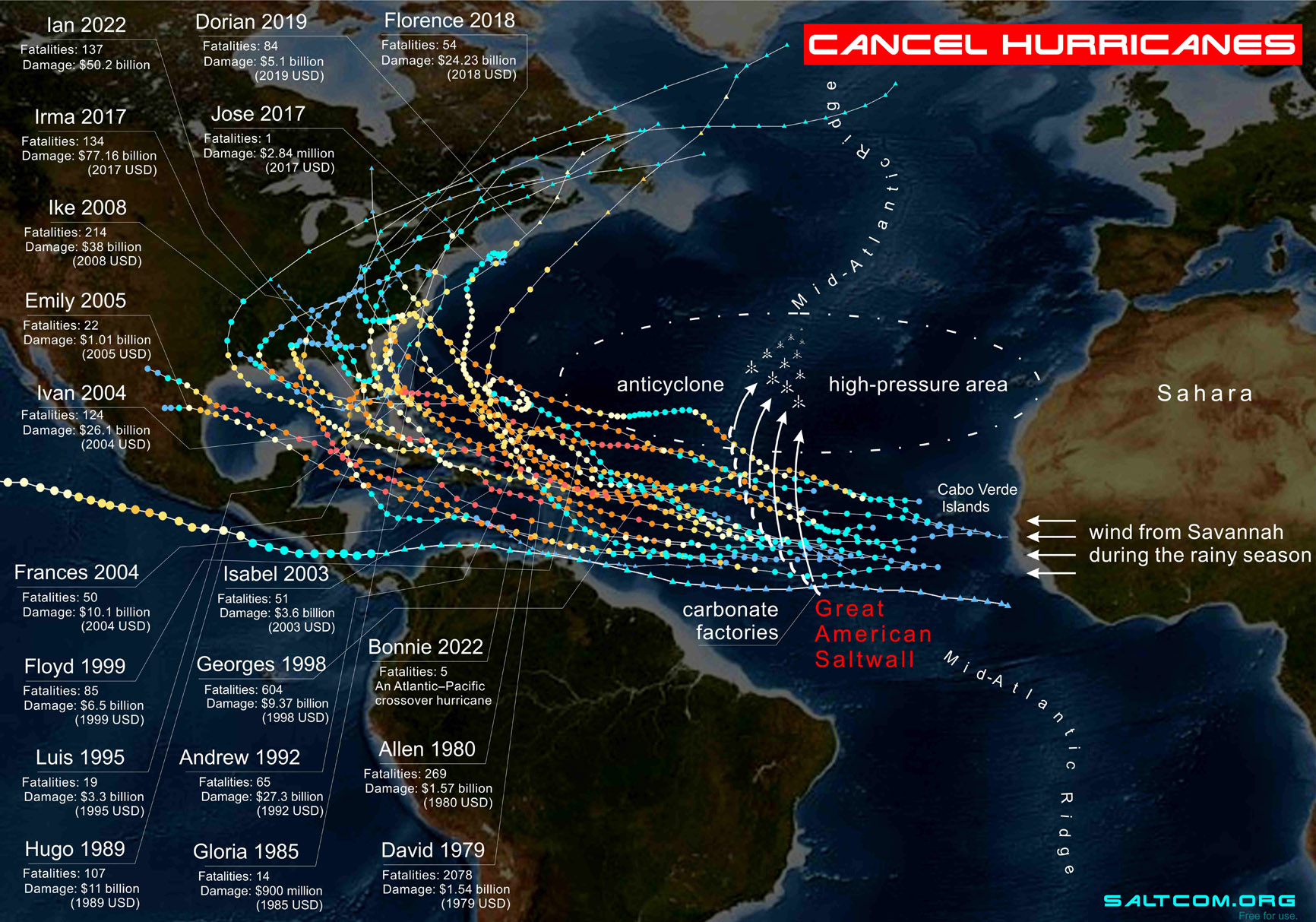
The systematic deviation of the initial pre-hurricanes, cyclones into the zone of anticyclone will lead to their “annihilation”. That is, the energy stored with moist air from African savannah will be directed to the large calm region of the Atlantic of about the 30s latitudes. During sailing navigation this area was called "horse latitudes" due to the long periods of calms.
On the salinity map, this area is an orange-red, high salinity zone 37-38 ‰. Here you need to understand that the satellite measures the salinity of 2 cm of the surface, while the bottom waters are everywhere in oceans less than 35 ‰, that is, by about 3 ‰ less.
The energy stored in a water vapor will dissipate through the waves in the deserted part of the Atlantic Ocean, contributing to the mixing of the surface layers, which means acceleration of the absorption of CO2 by the ocean from the atmosphere. The initial excess of energy can be directed to useful work instead of natural disasters, damage and deaths.
Above the desalinated waters at the same temperature, the vapor pressure is higher, the air has greater heat capacity and internal energy. This energy supply and attract a hurricane.
For example, Katrina 2005 is the most destructive hurricane in the history of the USA, despite the short track inside the Mexican Gulf, quickly gained strength to the 5th category, actually moving along the gradient of salinity to the mouth of the Mississippi River. In the distance of critical hurricane amplification to the highest category, the surface salinity decreased from 40 to less than 1 ‰ of river water, that is, by 4 %, and, accordingly, vapor pressure as a function of salinity increased by approximately on 0.08 kPa or about 2,2 %, that is almost on half of salt. Gibbs Free Energy of seawater increased by about on 1 kJ/kg or almost on 25 % with maximum on 10 ‰ of salinity.
Knowing thermophysical property data of seawater with reference points in depth (0, 714 , 1224 m. from MIT) we can build a 3D map of movement of energy actually online. Using data from spacecraft and a international Argo program, it is now possible to know everything about the oceans and all water.
Here we note that Mississippi carries fresh water above huge underground deposits of rock salt, the thickness of which is several hundred and thousands of meters. Moreover, massive layers of salt with diapirs are located directly under the mouth of the river, New Orlean and under the track of Katrina on the desalinated surface of the Mississippi river flow in the Gulf...
Speaking of the Latin proverb: "sapienti sat".
Similar weather patterns occur on the west of the Pacific Ocean. Hurricanes here are called typhoons. They also reach high power and even more lethality. For example, Typhoon Haiyan 2013 or Super Typhoon Yolanda killed more than 8 thousand people.
The map shows the track of Typhoon Meranti 2016, which killed "only" 47 people and caused $4.79 billion in damage, and a scheme of how this could have been prevented.
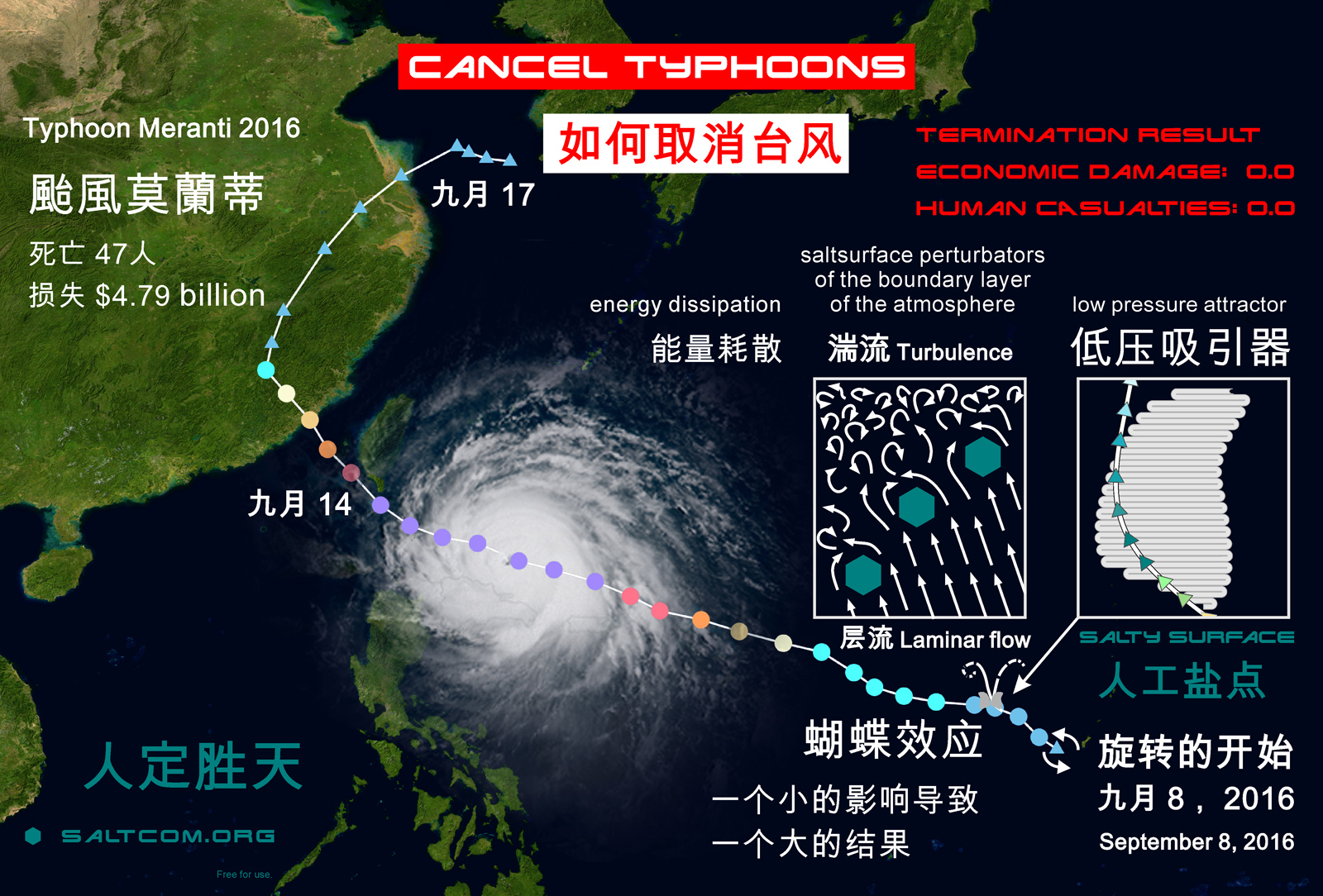
The situation in the region is aggravated by a significant desalination of the surface water of the Pacific Ocean (see salinity map↑) due to the large continental runoff from Eurasia and Global Atmospheric Circulation . The salinity of the ocean surface here is much lower than the average world – about 29-32 ‰. Accordingly, higher air humidity. At a standard temperature and pressure, moist air at maximum saturation has a density of 28.51 g/mol, while under the same conditions the average molar mass of air is 28.57 g/mol. Moist air has more buoyancy, it rise up like bubbles in boiling water.
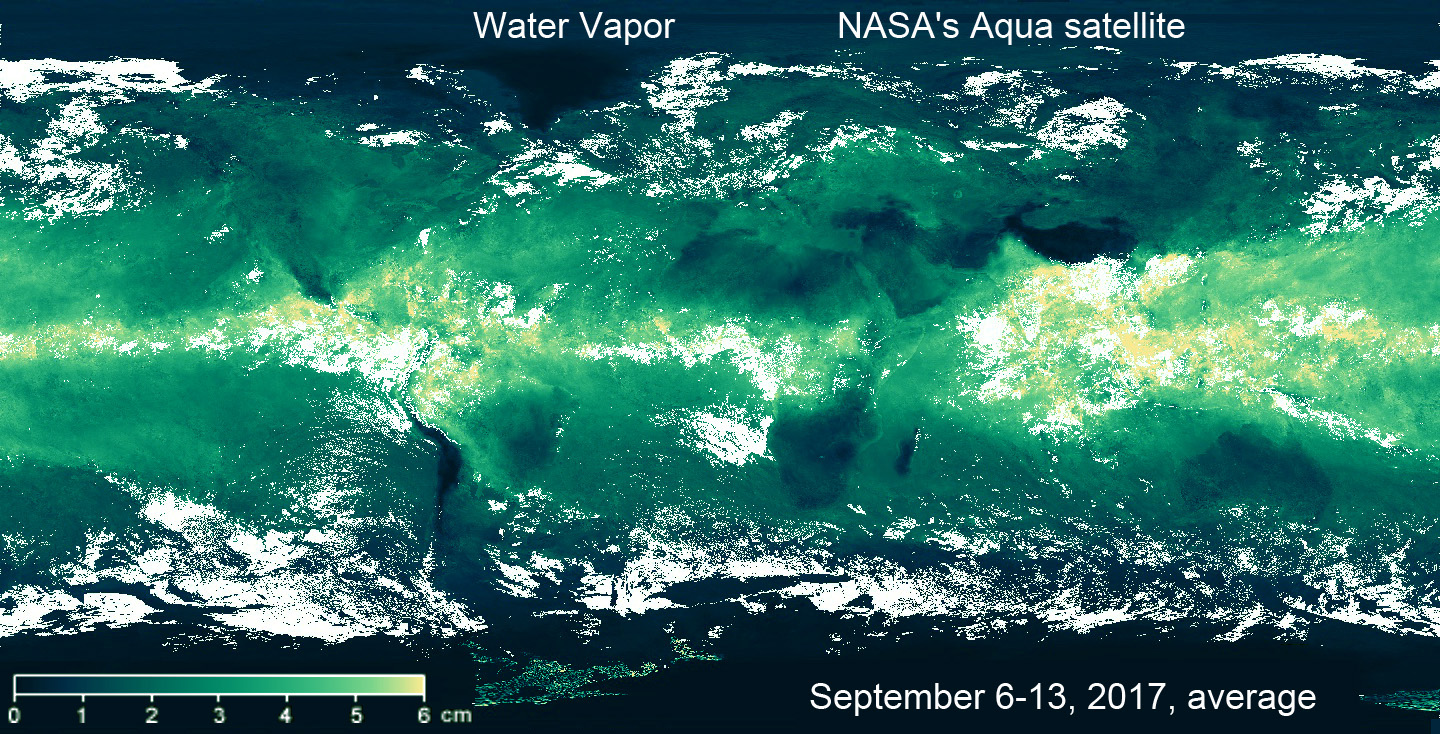
If we look at the water vapor map, we can see a rather motley picture of atmospheric moisture in this region. Due to the relief of the bottom, islands and currents, here in the late summer and autumn the heated ocean regularly forms some “a boiler”, generator and amplifier of typhoons. This season is dominated by winds that blow from the warm ocean to the mainland and carry these turbulent masses of atmospheric water to the densely populated coast. It is believed that the season of typhoons is inevitable.
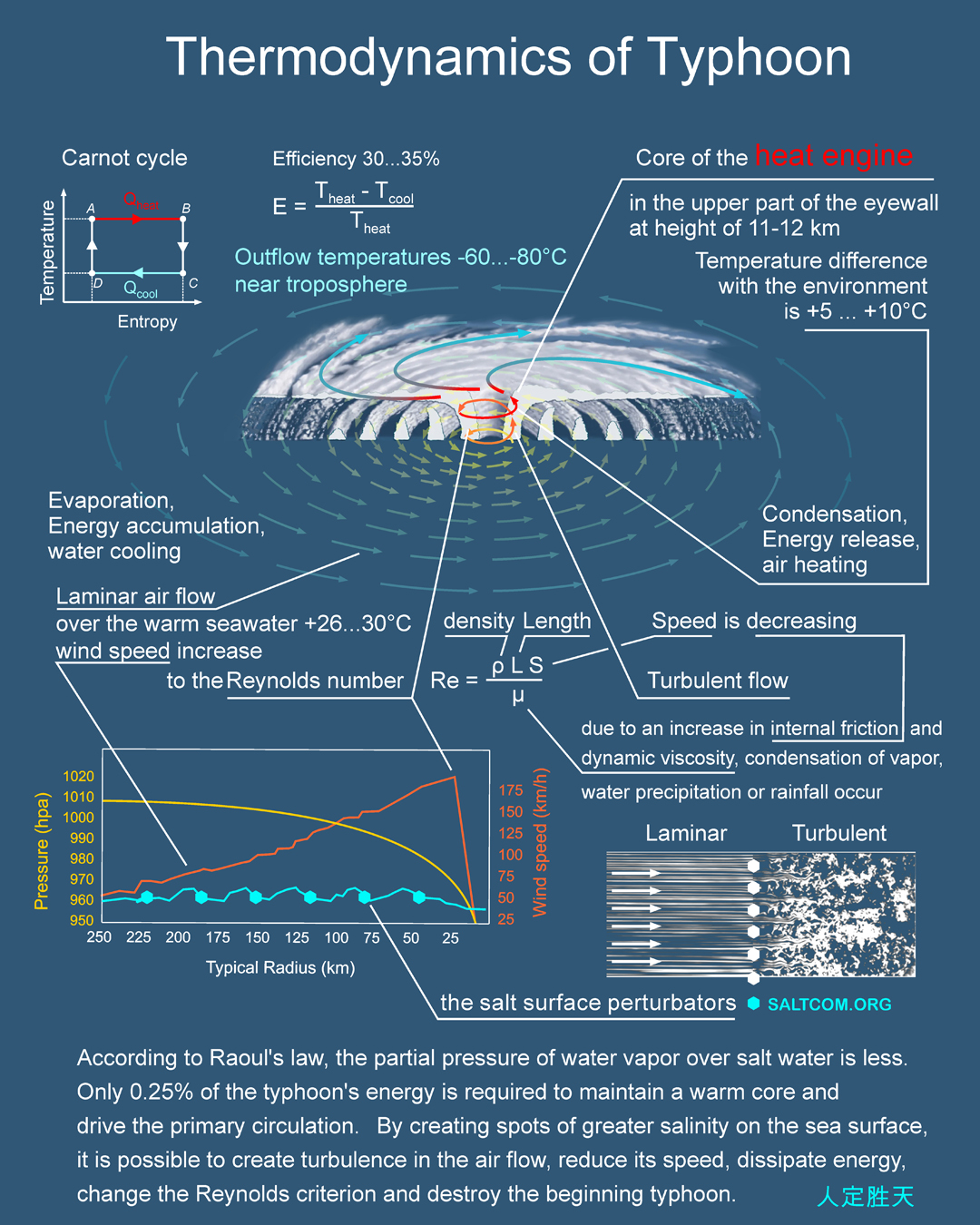
But every cook knows that if to add salt to a boiling soup, the boiling will decrease or stop.
The difference between a Pacific pool and a soup pot is in the degree of impact. When talking about global warming or ocean acidification, this refers to a thin layer near the surface ± several tens of meters. Whereas the depth of the oceans is much greater. The average depth of the World Ocean is 3688 m, and the Pacific Ocean is even more – 4280 m. That is, the changeable part is very small, its size is three orders of magnitude smaller than the unchangeable part. Even if all the fossil fuels were mined and burned, and all the biomass carbon was oxidized to CO2 and dissolved in seawater, this would have very little effect on the total carbon content in the ocean (see Size of carbon reservoirs on the Earth ↑).
On the contrary, if the huge reserves of salt on land, underground and under the ocean are dissolved in seawater, then it is theoretically possible to increase its salinity by two and a half times to 82 ‰, make a “new Cretaceous period” and chemically precipitate all carbon into carbonate minerals.
So, in order to increase the salinity by 2-4‰ of the upper 10 m of water for 1 km2, only 20-40 thousand tons of salt are needed, the amount comparable to the deadweight of one ship. This regional anti-typhoon system will require a fleet of several hundred ships, what is possible already now.
Given that the salt diffusion into the lower layers takes months or years, conditionally, a month is always more than the presence of vapor in the atmosphere, which is limited to 9 days, then the game of people with the boundary of seawater and atmosphere, a kind of typhoon cancellation game that can continue for centuries. The game in which humanity will always win over nature.
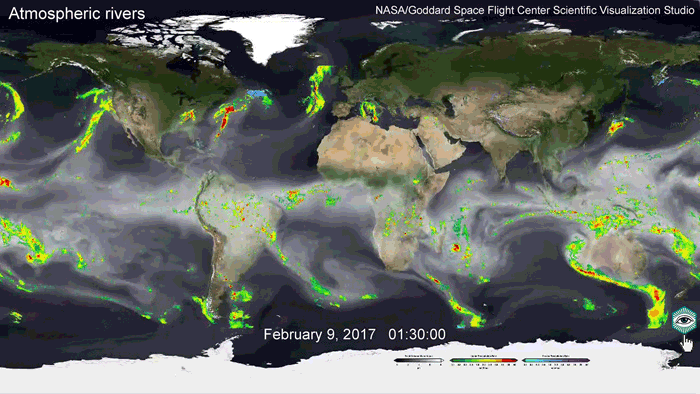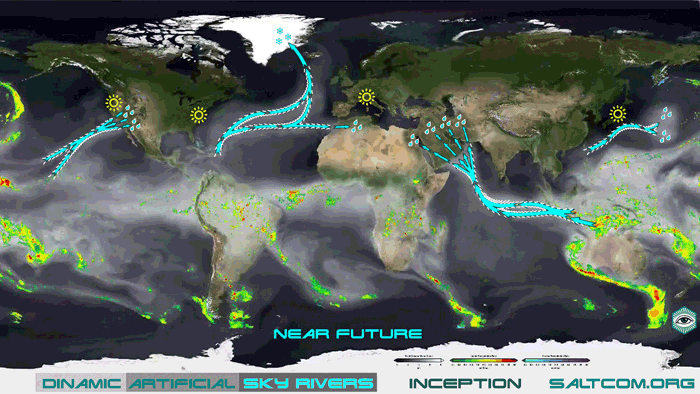
But for now, people are very vulnerable. For example, the surface of the Indian Ocean is very desalinated, especially the northern and eastern parts, 25-28 ‰. High humidity regularly torments all coasts with disasters. So Cyclone Bhola 1970 passed through the semi-fresh Bay of Bengal, gained strength, entered the mouth of the Ganges river, killed 0.5 million (!) people, and caused the formation of a new state of Bangladesh.
Many climatic and weather problems can be solved with salting technology. It is possible to cancel not only hurricanes and cyclones, but also to destroy stable anticyclones, sources of abnormal heat and forest fires. It will also be possible to cancel other natural disasters: heavy rains, hail and snowfalls, storm surge, floods and mudflows, extreme heatwave, drought and desertification.
Extreme events are not obligatory in nature. Нumanity is already ready to make its home comfortable.
Once the technology reaches a certain scale, when the CO2 sequestration becomes widespread, it will be possible to control the movement of water in the atmosphere through artificial sky rivers or vapor canals. Such hydro engineering can be more cost-effective than the construction of land-based water canals, more dynamic, more flexible, more environmentally friendly, more scalable and more functional. Thus, the excess atmospheric waters of the Indian Ocean can be dynamically directed to irrigation of the Arabian deserts, contribute to the softening of the climate of the region and the transformation of lifeless sands into flowering gardens.
 You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
Each nexus from mining, preparation, storage and transportation of salt to the final dissolution in seawater is a block that is connected to a system of similar blocks in other places in the world. This global system is distributed and has many independent participants that are interconnected through mathematical dependence on the fundamental laws of nature (the "golden polynomials" mentioned above).
It is a blockchain system with technologies similar to digital currencies. It will implement the same elements as a distributed timestamp server, a simplified a proof-of-work system (not Adam Back's Hashcash but with core certification), payment verification, etc.
But unlike Satoshi Nakamoto, we exclude interest revenue. Investors who care about income ethics or follow religious laws will be able to invest for the benefit of themselves and all people. Since profit is generated in the real sector of the economy and occurs on a contractual basis. There is also no need to hide the participants from each other, fight against secret cracksmans and make huge meaningless calculations that devour huge energy resources and pollute the planet.
We are creating a new alternative currency — the SALTCOIN, which is not limited by either the size of the total money supply or the time limit. The number of coins will always grow in the foreseeable future (hundreds of years), because humanity will emit CO2 for a long time. In fact, this emission is the emission of money, which is not carried out by the Central Bank of a particular country or an energy-intensive algorithm, but by nature itself and all of humanity.
Stability
SALTCOIN is not subject to speculative fluctuations because, unlike all other money, it has "intrinsic value". Its essence is a rigid connection between finance and laws of nature. Salt cannot lose its power, "to become unsalted" is a figure of speech, a biblical metaphor. There are laws of conservation of energy, conservation of mass, and stoichiometry.
No inflation
SALTCOIN has an intrinsic value that is created at the moment of dissolution and does not disappear over time. Salt once dissolved in the ocean will work always. The residence time of the main components that generate the process of carbonate precipitation is estimated in millions of years. So the residence time in the ocean of halogens Cl and Br is 100 million years, sodium Na - 47 million, boron B - 20, Mg - 13 million and Ca - 1 million years.
No currency in the world can compare with such a time of circulation.
Long term growth
It is clear that the CO2 sequestration market will to grow in the near and far future. Comparing the current world salt production (270 million tons) with the required volume of tens and hundreds of gigatonnes, billions of tons, we will see a thousandfold potential for market growth.
SALTCOIN will become both a secure store of value and a highly moral source of income.
Humanism
Personally, we do not plan to receive super profits from our developments. We are guided by the Humanist Manifesto and believe that
"Working to benefit society maximizes individual happiness."
Saving the world from an impending climate catastrophe is a noble enough and attractive goal. We are sure that we will find our supporters, people who are not indifferent to the future of their children, people who think globally and want to give the Earth to posterity better than they received it.

Obviously, a big money will come here for a long time. In your opinion, how big?
 You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
You are viewing a simplified version for mobile devices. To open the full version for computer, click on the icon.
![]() All texts and images are free to use. You can copy, paste, save, edit, modify, send, receive, repost, accept, transfer, sell, buy, to profit, agree, dispute, develop, ignore, take to heart, do anything with them without restriction. Freeedom!!!
All texts and images are free to use. You can copy, paste, save, edit, modify, send, receive, repost, accept, transfer, sell, buy, to profit, agree, dispute, develop, ignore, take to heart, do anything with them without restriction. Freeedom!!!